The pathway ALL uses to enter into the nervous system.
Scientists have uncovered how acute lymphoblastic leukemia (ALL) cells invade the central nervous system (CNS), a relatively common occurrence that makes the cancer immeasurably more difficult to treat. Despite being a characteristic across ALL subtypes, the mechanism of how the CNS invasion occurs remains unknown.
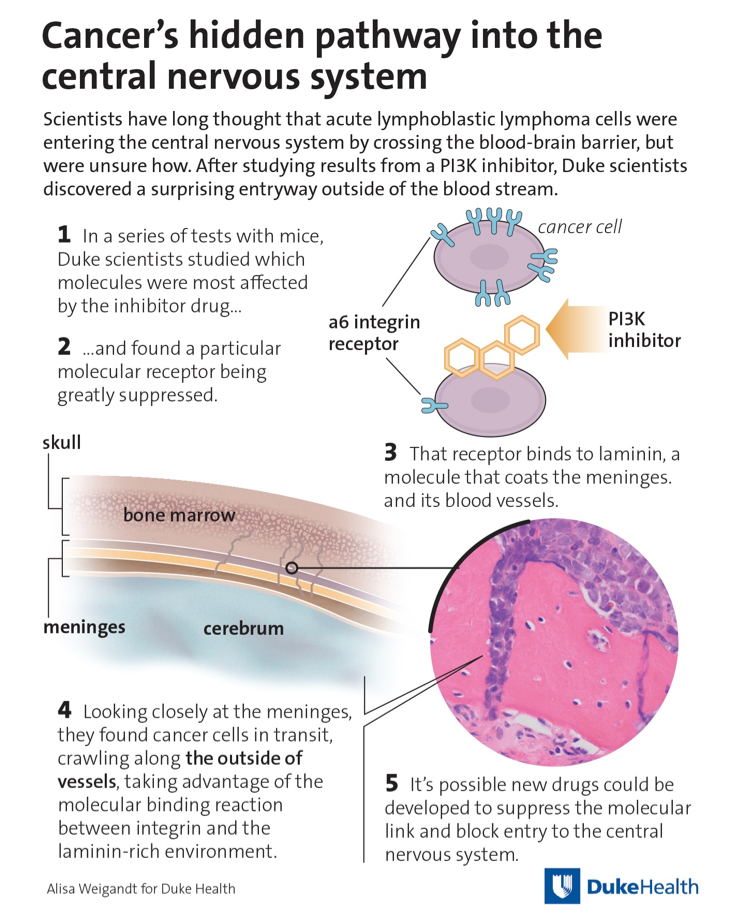
The researchers used three different mouse models of ALL to study how human leukemia cells spread into the CNS. It has long been thought that these cells enter the CNS by crossing the blood–brain barrier (BBB), although the details of how this happened remained undetermined. The researchers' work focused on PI3K, a key regulator of signaling pathways needed for growth, survival, and invasion by cancer cells. Published in Nature, the study is entitled, “Leukaemia hijacks a neural mechanism to invade the central nervous system.”
Upon showing that the ALL cells are unable to breach the BBB in mice, the researchers, led by Dorothy Sipkins, M.D., Ph.D., associate professor at Duke University Medical Center, looked at other approaches that could be taken by the cells to enter the CNS. Their data suggest that the ALL cells co-opt a pathway that involves contact between integrins expressed on the ALL cells and the basement membrane of these vessels. More specifically, they show that the cells migrate into the CNS along vessels that pass directly between vertebral or calvarial bone marrow and the subarachnoid space.
“It's a very unexpected way for cells to travel into the central nervous system,” said Dr. Sipkins.
Their discovery came when the researchers gave the ALL mice a molecule called GS-649443, a known PI3K inhibitor. The treated animals had reduced signs of cancer invasion of the CNS when compared to mice that did not receive the molecule—the ALL cells remained in their bone marrow. This suggested that the mode of action of the drug, the inhibition of PI3K, specifically decreased the ability of ALL cells to enter the CNS.
The team went on to identify an integrin, the α6 integrin specifically, in the ALL cells that had decreased expression in response to PI3K inhibition. α6 integrin binds laminin, a component of the extracellular matrix that surrounds large blood vessels, the meninges, and the CNS choroid plexus to help facilitate cell migration. The laminin receptor, α6 integrin, is expressed in most cases of ALL. They found that α6 integrin–laminin interactions mediated the migration of ALL cells toward the cerebrospinal fluid in vitro.
Using microscopy, the researchers found that small blood vessels coated with laminin are sites with high levels of ALL cells, indicating that moving along these vessels may be how the cells reach the CNS. These blood vessels pass directly to the meninges tissue that lines the spinal cord and brain. The researchers found that the ALL cells were “grabbing onto the laminin surrounding these blood vessels and zipping down into the meninges region where cerebral spinal fluid circulates.”
“When we dissected the spines and the vertebrae that surround the spinal cord, we noticed this strange thing: You could see all the leukemia cells in the vertebral bone marrow and it looked like they were streaming through a channel at the bone surface straight into the spinal cord,” Dr. Sipkins said. “It just all made sense in what we see in patients, the anatomy, the molecular mechanisms. It was very exciting to piece together.”
The finding that cancer cells take over a route used for neural migratory pathfinding leads the authors to suggest that “Future studies may reveal that this unique ALL trafficking pathway is involved in immune surveillance or inflammatory processes. Exploring the interactions between normal and malignant immune cells and these vascular scaffolds may thus reveal multiple points of intervention to treat CNS invasive processes.”
Dr. Sipkins remarks that, “Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” and adds that GS-649443, or other molecules that work in a similar way, could have therapeutic benefit but would require more study. This new way of thinking about ALL invasion of the CNS could lead to novel approaches in drug design.

![Leukemia Blood Cell [Kateryna Kon/Science Photo Library/Getty Images] Leukemia Blood Cell [Kateryna Kon/Science Photo Library/Getty Images]](https://www.genengnews.com/wp-content/uploads/2018/08/Jul18_2018_Getty_685029693_LeukemiaBloodCell_6001216814959.jpg)
