A group of U.K. scientists have developed two new technologies for rapidly analyzing bioprocesses. The team, from University College London, has developed a “virus laser” and a currently unnamed system for close to real-time monitoring of products and impurities.
“This is very new work. We’ve presented results, but haven’t yet published a paper,” says John Hales, PhD, a research fellow from the department of biochemical engineering, who presented his work at the Bioprocessing Summit Europe in March.
Hales explains how the dye-labelled M13, when it flows into a laser photometer, generates a laser signal. Small changes in the probe concentration of 50% can lead to a 10,000 change in the output signal, he explains.
“So, what we’re looking to develop, and it is early stage, is a ligand-binding assay that could rapidly report on the concentrations of, say, product or impurities, ideally working at-line to the bioprocess,” continues Hale.
In 2020, Hales was awarded a £1.2 million UK Research and Innovation Future Leaders Fellowship to work on the virus laser.
The second technology, which is currently unnamed, aims to quantify proteins within mixtures in real-time without using labels, such as when proteins co-elute from chromatography columns.
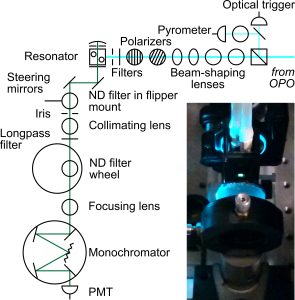
According to Hales, the technology consists of a short-pulse nanosecond laser that emits at a UV wavelength of 266nm. The pulse is steered towards a capillary in which proteins are flowing, for example, out of a chromatography column.
The proteins are excited, and their fluorescence captured by an ellipsoidal reflector and a photodiode, Hales explains. The team then uses a sampling oscilloscope to digitize the signal and data processing algorithms to, for example, identify the different proteins.
“You have a signal that you can measure, which is directly linked to the protein structure,” he says. “And you can use that like an optical signature.”
The researchers are currently spinning out a company, Roxijen, from the university. They are also working with industrial partners.






