Anton Simeonov Ph.D. National Institute of Health
Review of study points to relative abundance of PTMs in human cells.
ASSAY & Drug Development Technologies offers a unique combination of original research and reports on the techniques and tools being used in cutting-edge drug development. The journal includes a “Literature Search and Review” column that identifies published papers of note and discusses their importance. GEN presents one article that was analyzed in the “Literature Search and Review” column, a paper published in Science titled “Sirt5 Is a NAD-dependent protein lysine demalonylase and desuccinylase.” Authors of the paper are Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, and Lin H.
Abstract from Science
Silent information regulator 2 (Sir2) proteins (sirtuins) are nicotinamide adenine dinucleotide–dependent deacetylases that regulate important biological processes. Mammals have seven sirtuins, Sirt1 to Sirt7. Four of them (Sirt4 to Sirt7) have no detectable or very weak deacetylase activity. We found that Sirt5 is an efficient protein lysine desuccinylase and demalonylase in vitro. The preference for succinyl and malonyl groups was explained by the presence of an arginine residue (Arg105) and tyrosine residue (Tyr102) in the acyl pocket of Sirt5. Several mammalian proteins were identified with mass spectrometry to have succinyl or malonyl lysine modifications. Deletion of Sirt5 in mice appeared to increase the level of succinylation on carbamoyl phosphate synthase 1, which is a known target of Sirt5. Thus, protein lysine succinylation may represent a posttranslational modification that can be reversed by Sirt5 in vivo.
Commentary
The report of Du and colleagues highlights the discovery and initial characterization of yet another protein posttranslational modification (PTM). During the past decade, the number and chemical diversity of PTMs have grown tremendously, with protein acetylation, methylation, ubiquitinylation, and multiple other changes being added to the classical PTM, protein phosphorylation. Sirtuins were originally characterized as NAD-dependent deacetylases, with Sirt1 being the most prominent member of the family. However, some of the more recently isolated sirtuins have displayed very poor enzymatic activity against acetylated protein substrates, complicating the studies of their biological function.
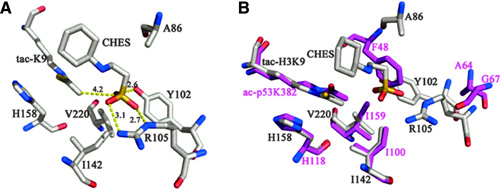
Figures 1A and 1B. The structure of Sirt5 revealed an unusual acyl pocket. (A) The acyl pocket of Sirt5 was partially occupied by the sulfate from the N-cyclohexyl-2 aminoethanesulfonic acid (CHES) molecule via interactions with Arg105 and Tyr102. The sulfur was 4.2 Å away from the thioacetyl group. (B) Alignment of Sirt5-thioacetyl peptide structure (gray) and Sir2Tm-acetyl peptide structure (PDB 2h4f, magenta)
In the present study, the authors focused on Sirt5 and, by performing a comparative analysis of its crystal structure, concluded that an acyl group bearing a negatively charged carboxylate should bind to Sirt5 better than a regular acetyl group does, indicating that a dicarboxylate may be a good candidate PTM that Sirt5 operates on. Malonyl-CoA and succinyl-CoA were considered as the obvious candidate precursors due to their relative abundance and utilization in several metabolic processes. Histone H3-derived peptides carrying succinyl and malonyl substitutions of Lysine 9 (H3K9-Su and H3K9-Ma) were tested with several sirtuins, with acetyl counterparts being used as controls (Figures 1A, 1B, 1C, and 1D).
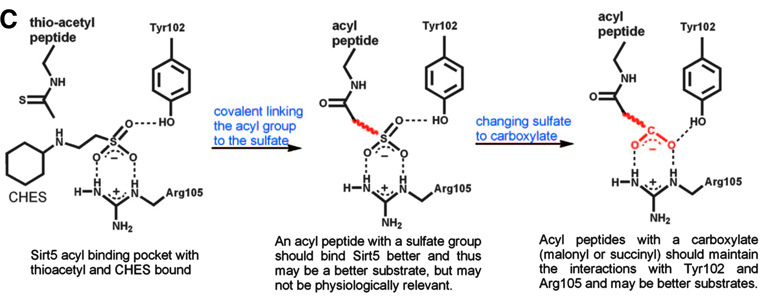
Figure 1C. The rationale for predicting that malonyl/succinyl peptides could be better substrates for Sirt5.
While Sirt1 efficiently catalyzed the deacetylation of the H3K9-Ac substrate, as expected, Sirt5 almost did not process the acetyl substrate at all and instead showed high efficiency against H3K9-Su and H3K9-Ma. Subsequent X-ray crystallography analysis confirmed the engagement of the succinyl and malonyl substrates into the Sirt5 active site, as predicted from the initial comparative analysis.
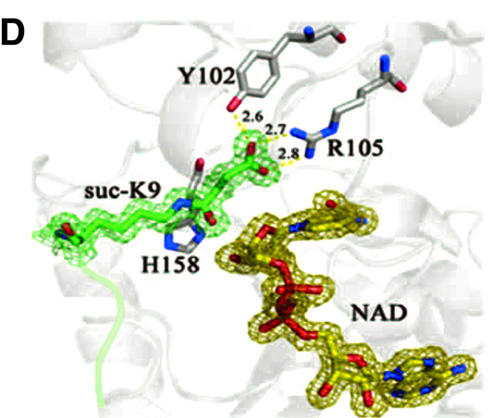
Figure 1D. Sirt5-succinyl peptide-NAD ternary structure showing that the succinyl group interacted with Tyr102 and Arg105
Using the radiolabeled substrate 32P-NAD, which can form 32P-labeled O-Ma-ADP-ribose and O-Su-ADP-ribose, and treating bovine liver mitochondrial peptides with it and Sirt5, the authors demonstrated the presence of succinylated lysines in mitochondrial proteins (Figures 2A-2H).
Figures 2A and 2B. (A) Succinyl lysine was detected in bovine liver mitochondria. Sirt5-catalyzed hydrolysis of malonyl and succinyl peptides could be detected by using 32P-NAD, which formed 32P-labeled OMa-ADPR (lane 2) and O-Su-ADPR (lane 3). No reaction occurred with acetyl peptide (lane 1). The formation of O-Ac-ADPR catalyzed by Sirt1 was detected (lanes 4 and 8). O-Su-ADPR was formed when bovine liver mitochondria peptides were incubated with Sirt5 (lane 9) but not with Sirt1 (lane 10). The control with BSA peptides and Sirt5 did not generate O-Su-ADPR (lane 12). CD38-catalyzed hydrolysis of NAD was used to generate the standard 32PADPR spot (lane 13). (B) The CPS1 activities were measured by using the liver lysates from Sirt5 wild-type and Sirt5 KO mice. (n = 3 pairs of mice, p < 0.05).
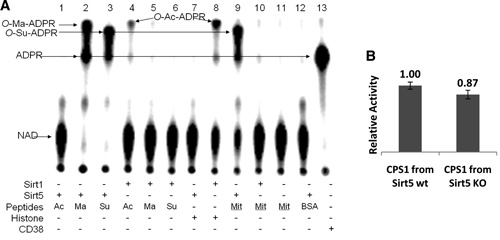
Figures 2A and 2B
The in vivo relevance of these new PTMs was further demonstrated through affinity capture and purification of succinyl peptides from bovine liver mitochondria via the use of a FLAG-tagged Sirt5, and subsequent protein identification using liquid chromatography and mass spectrometry; by use of a similar approach, malonyl lysine residues were also identified on several proteins. Lastly, a Sirt5 knockout experiment demonstrated that protein lysine succinylation may be a type of PTM that is reversed by the action of Sirt5 in vivo. The relative abundances of these new PTMs in human cells and tissues, as well as their role in human biology, await further elucidation.
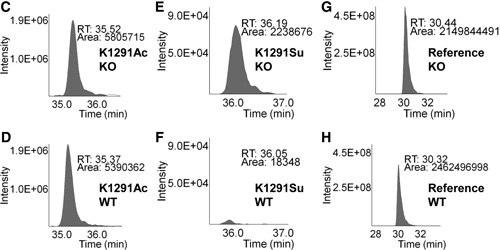
Figures 2C–2H. Relative quantitation analysis was achieved by extracted ion chromatograms (XICs) for peak areas of CPS1 K1291 acetyl peptides from (C) Sirt5 KO mice and (D) wild-type mice; of CPS1 K1291 succinyl peptides from (E) Sirt5 KO mice and (F) wild-type mice, and of a reference peptide from (G) Sirt5 KO mice and (H) wild-type mice.
For a discussion about the impact of PTMs on the structure and function of therapeutic antibodies, click here.
Anton Simeoniv works at the NIH.



