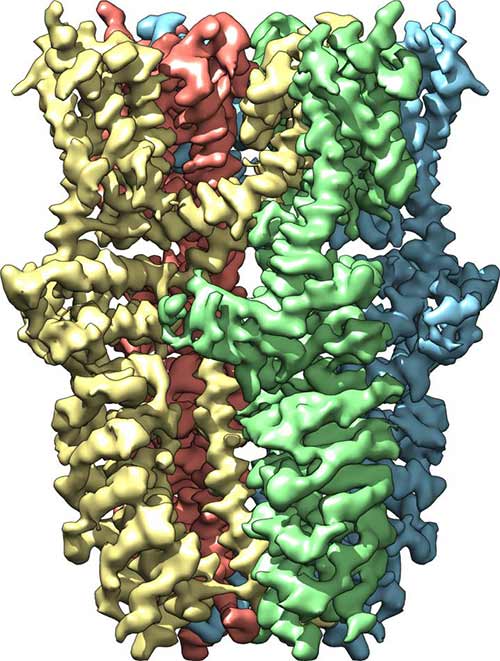
Side view of the TRPA1 receptor shows that it is composed of four subunits, colored yellow, green, blue, and red. When the receptor is activated by wasabi or certain other stimuli, channel opens from outside the cell (top) to inside the cell (bottom).
Scientists at the University of California, San Francisco (UCSF) have managed to resolve the 3D structure, to near-atomic resolution, for the ion channel that has been informally dubbed the wasabi receptor. Resolving the protein structure to this level of detail could assist scientists in developing new anti-inflammatory compounds to treat and manage pain.
The ion channel observed in this study, officially named TRPA1, belongs to a group of plasma membrane spanning channels that evolved to detect chemical agents that typically act as irritants—substances that range from wasabi to capsaicin (the molecule that gives heat to chili peppers) to tear gas. Additionally, these channels can also be triggered by pain signals that originate within the cell, often in response to tissue damage and inflammation.
“The pain system is there to warn us when we need to avoid things that can cause injury, but also to enhance protective mechanisms,” said David Julius, Ph.D., professor and chair of the Department of Physiology ay UCFS and co-senior author of the current study. “We've known that TRPA1 is very important in sensing environmental irritants, inflammatory pain, and itch, and so knowing more about how TRPA1 works is important for understanding basic pain mechanisms. Of course, this information may also help guide the design of new analgesic drugs.”
The findings from this study were published recently in Nature through an article entitled “Structure of the TRPA1 ion channel suggests regulatory mechanisms.”
Using a technique called electron cry-microscopy (cryo-EM), where the proteins are hit with a stream of electrons at extremely low temperatures, Dr. Julius and his colleagues were able to visualize the 3D structure of TRPA1, including a small cleft on protein where an experimental new drug binds to the channel.
“A few drugs have been developed that target TRPA1, and in our 3D structure we can see where one such drug binds,” explained Dr. Julius. “This provides important insight into how this one major class of drugs interacts with TRPA1 and thus how it may work to block channel function.”
The UCSF team was able to use the cryo-EM technique thanks to some recent advances in hardware and imaging software, which allowed them to visualize TRPA1 at a resolution of 4 angstroms. To put that into perspective, the width of a human hair is about 500,000 angstroms.
Traditionally, these types of high-resolution protein images are done using X-ray crystallography. However, this technique is not only notoriously difficult and time-consuming, there has yet to be a membrane protein, like TRPA1, that has been successfully crystallized. The researchers needed to find a better solution.
“We came at it from a different angle,” said Yifan Cheng, Ph.D., associate professor of biochemistry and biophysics at UCSF and co-senior author on the study. “Since crystallization was so difficult, we decided to try cryo-EM, which bypasses this requirement.”
The UCSF team was able to obtain images of the TRPA1 ion channel in three different states: closed, open, and partially open. This allows investigators a range of views for gathering insight into the channels inner workings, but Dr. Julius and his team are optimistic that they will be able to generate even more images of TRPA1 in additional conformations.
“Cryo-EM has undergone a resolution revolution that has enabled us to literally see TRP channels in all their glory,” Dr. Julius stated. “We've had some idea what TRPA1 might look like, but there's something elegant and satisfying about obtaining the structure, because seeing really is believing.”