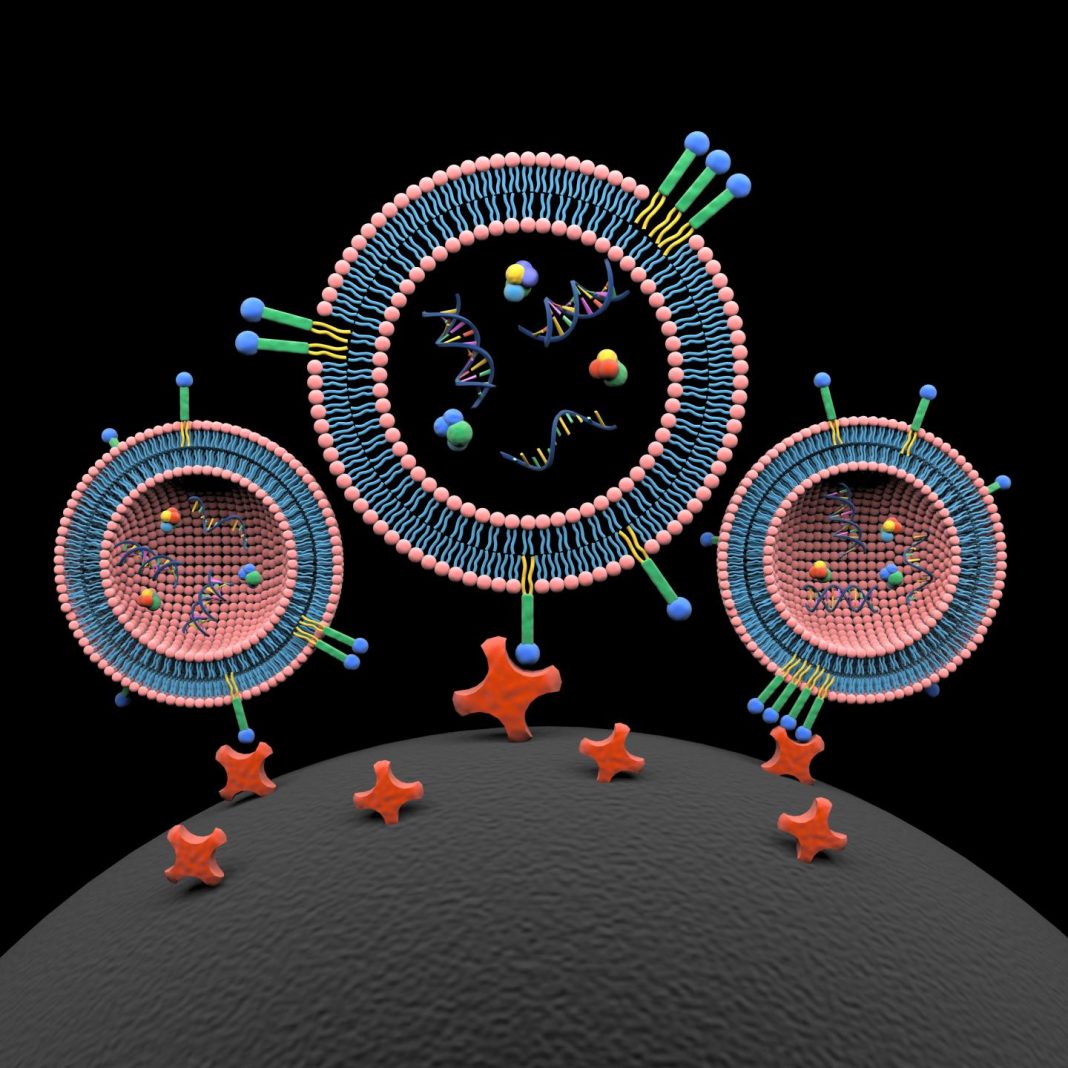
![Lipid nanoprobes (blue, green, and yellow) spontaneously insert into lipid bilayer of three extracellular vesicles (EVs). The cargo content of EVs includes proteins, DNA, and RNA. The lipid nanoprobe-labeled EVs are captured onto the surface of a magnetic bead (black, at bottom) through interaction with conjugated avidin molecules (red). Exosome isolation and exosome cargo analysis offer new opportunities for a diverse range of molecular analyses, including mutation detection from blood plasma of cancer patients.[Xin Zou/Penn State]](https://genengnews.com/wp-content/uploads/2018/08/137646_web1122525120-1.jpg)
Lipid nanoprobes (blue, green, and yellow) spontaneously insert into lipid bilayer of three extracellular vesicles (EVs). The cargo content of EVs includes proteins, DNA, and RNA. The lipid nanoprobe-labeled EVs are captured onto the surface of a magnetic bead (black, at bottom) through interaction with conjugated avidin molecules (red). Exosome isolation and exosome cargo analysis offer new opportunities for a diverse range of molecular analyses, including mutation detection from blood plasma of cancer patients.[Xin Zou/Penn State]
If we were able to actively watch cells at the nanoscale level, an entirely new world of molecules and processes would provide us with as many new questions, as well as answers to long-standing hypotheses. Yet, if our attention were focused on the cell membrane, we would see active secretion of extracellular vesicles (EVs), ranging in size from 30 to 50 nanometers (nm) in diameter. These tiny lipid sacks were once thought of as cellular garbage dumps; but in recent years, scientists have discovered that these nanoparticles contain valuable information about cellular health and disease, especially for cancer. However, isolating EVs has always been a challenge for researchers.
Now, a team of investigators at Penn State University (PSU) has developed nanoprobes to rapidly isolate these EVs, with the hope of developing precision cancer diagnoses and personalized anticancer treatments. The findings from this new study were published recently in Nature Biomedical Engineering in an article entitled “Rapid Magnetic Isolation of Extracellular Vesicles via Lipid-Based Nanoprobes.”
“Most cells generate and secrete EVs, but they are difficult for us to study,” explained senior study investigator Si-Yang Zheng, Ph.D., associate professor of biomedical engineering and electrical engineering at PSU. “They are submicrometer particles, so we really need an electron microscope to see them. There are many technical challenges in the isolation of nanoscale EVs that we are trying to overcome for point-of-care cancer diagnostics.”
Recently researchers have come to understand that EVs often contain double-stranded DNA, RNA, and proteins that are responsible for communicating between cells—as well carrying identification markers as to their origin cells, including tumor cells. In the case of cancer, at least one function for EVs that has been observed previously is to prepare distant tissue for metastasis.
In the current study, the PSU team set out to develop a method for isolating and purifying EVs in blood samples that contain multiple other components. This type of isolation falls into the burgeoning realm of liquid biopsy for cancer diagnosis—which offers numerous benefits over traditional invasive techniques to extract cancer cells. For instance, in lung cancer or brain cancers, such invasive techniques are difficult, expensive, and can be painful.
“Noninvasive techniques such as liquid biopsy are preferable for not only detection and discovery, but also for monitoring treatment,” noted so-senior author Chandra Belani, M.D., professor of medicine and deputy director of the Cancer Institute at Penn State College of Medicine.
For the new method, the researchers created surface-modified magnetic beads that are 400 to 500 nm in diameter, with labeling probes that are on the order of 10 nm. When the system is optimized, the researchers can isolate the EVs from blood plasma samples in about 15 minutes. The EVs and their contents can then be analyzed in a lab setting or sent to a commercial diagnostic lab to characterize the DNA, RNA, and proteins.
“We invented a system of two micro/nanomaterials,” Dr. Zheng remarked. “One is a labeling probe with two lipid tails that spontaneously insert into the lipid surface of the EV. At the other end of the probe, we have a biotin molecule that will be recognized by an avidin molecule we have attached to a magnetic bead.”
Dr. Zheng and his team used their newly developed nanoprobes to capture EVs from the blood plasma of patients with non-small-cell lung cancer.
“Aided by this new approach, we successfully isolated EVs from 19 patients with advanced lung cancer and identified DNA mutations that can guide precision therapy instead of routine chemotherapy,” stated lead study investigator Yuan Wan, Ph.D., a postdoctoral scholar in Dr. Zheng’s laboratory. “From collecting blood to obtaining EV-derived DNA, the whole procedure can be completed within 1 hour. It only requires a magnet and a common benchtop centrifuge. Compared to prevalent methods, the nanoprobe system would greatly facilitate clinical laboratory examination.”
Since this new technique requires only a blood sample and an external magnet, it is relatively inexpensive and fast compared to the current gold standard of separation—ultracentrifugation, which requires expensive equipment and takes hours to complete. Once validated in a larger study, the PSU researchers believe that this technique could be applied not only to lung cancer but also to most if not all solid tumors, which are responsible for 80% to 90% of cancer deaths.
“Sequencing the DNA isolated from the EVs will serve as a promising tool to track cancer evolution and monitor tumor dynamics with the ultimate goal of improving cancer survival,” Dr. Belani concluded.