![This composite image shows a dead female <i>Anopheles gambiae</i> mosquito and <i>Metarhizium pingshaense</i>, which has been engineered to produce spider and scorpion toxins. The fungus is also engineered to express a green fluorescent protein for easy identification of the toxin-producing fungal structures. [Brian Lovett/University of Maryland]” /><br />
<span class=](https://genengnews.com/wp-content/uploads/2018/08/142917_web2153091601-1.jpg) This composite image shows a dead female Anopheles gambiae mosquito and Metarhizium pingshaense, which has been engineered to produce spider and scorpion toxins. The fungus is also engineered to express a green fluorescent protein for easy identification of the toxin-producing fungal structures. [Brian Lovett/University of Maryland]
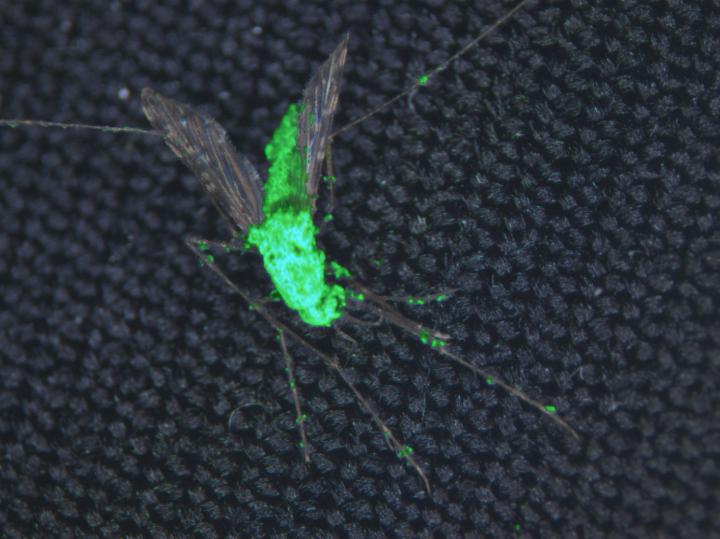
This composite image shows a dead female Anopheles gambiae mosquito and Metarhizium pingshaense, which has been engineered to produce spider and scorpion toxins. The fungus is also engineered to express a green fluorescent protein for easy identification of the toxin-producing fungal structures. [Brian Lovett/University of Maryland]
Summertime and mosquitoes go hand in hand, unfortunately. While most of us sit and read this from the comfort of our air-conditioned spaces, barely giving the little pests a second thought, much of the world still has difficulty in controlling the insects and the diseases they spread—like malaria, dengue, and Zika. In the case of malaria, which kills roughly half a million people annually, the parasite-carrying mosquitoes have become resistant to most traditional chemical insecticides, complicating efforts to fight the disease.
Now, a new study from the University of Maryland (UMD) and colleagues from Burkina Faso, China, and Australia suggests that a mosquito-killing fungus genetically engineered to produce spider and scorpion toxins could serve as a highly effective biological control mechanism to fight malaria-carrying mosquitoes. The fungus is specific to mosquitoes and does not pose a risk to humans. Moreover, the new study results suggest that the fungus is also safe for honey bees and other insects. Findings from this study were published recently in Scientific Reports in an article entitled “Improved Efficacy of an Arthropod Toxin Expressing Fungus against Insecticide-Resistant Malaria-Vector Mosquitoes.”
“In this paper, we report that our most potent fungal strains, engineered to express multiple toxins, are able to kill mosquitoes with a single spore,” explained study co-author Brian Lovett, a graduate student in the UMD Department of Entomology. “We also report that our transgenic fungi stop mosquitoes from blood feeding. Together, this means that our fungal strains are capable of preventing transmission of disease by more than 90% of mosquitoes after just five days.”
In the current study, the investigators used the fungus Metarhizium pingshaensei, which is a natural killer of mosquitoes. The fungus was originally isolated from a mosquito, and previous evidence suggested that the fungus is specific to disease-carrying mosquito species, including Anopheles gambiae and Aedes aegypti. When spores of the fungus come into contact with a mosquito's body, the spores germinate and penetrate the insect's exoskeleton, eventually killing the insect host from the inside out.
However, previous work showed that, on its own, the fungus requires fairly high doses of spores and a large amount of time to have lethal effects. To boost the fungus' deadly power, the researchers engineered the fungus with several genes that express neurotoxins from spider and scorpion venom—both alone and in combination with other toxins. The toxins act by blocking the calcium, potassium, and/or sodium channels required for the transmission of nerve impulses.
To mimic a real-world scenario, the research team captured wild, insecticide-resistant mosquitoes in Burkina Faso. Each engineered strain killed mosquitoes more quickly and efficiently than the unaltered fungus. But the most effective strain used a combination of two toxins, one derived from the North African desert scorpion Androctonus australis and another derived from the Australian Blue Mountains funnel-web spider Hadronyche versuta. The scorpion toxin blocks sodium channels, while the spider toxin blocks both potassium and calcium channels. Both of these toxins have already been approved by the U.S. Environmental Protection Agency for insecticidal use.
“The World Health Organization has identified insecticide resistance as the major threat to effective mosquito control, which is relevant not only to malaria but also to a number of mosquito-borne diseases, such as dengue, yellow fever, viral encephalitis, and filariasis,” noted senior study investigator Raymond St. Leger, Ph.D., professor in the UMD department of entomology and senior author of the study. “Unlike chemical insecticides that target only sodium channels, many spider and scorpion toxins hit the nervous system's calcium and potassium ion channels, so insects have no pre-existing resistance.”
In an attempt to ensure the safety of the inserted genes, the researchers included an additional failsafe mechanism—a highly specific promoter sequence, or genetic “switch,” which ensures that the toxin genes can only be activated in the blood of insects. As a result, the fungus will not release the toxin into the environment.
“The toxins we're using are potent but totally specific to insects,” Dr. St. Leger stated. “They are only expressed by the fungus when in an insect. Additionally, the fungus does nothing at all to bees and other beneficial species. So, we have several different layers of biosecurity at work.”
Encouraged by the results of the current study, the researchers plan to expand their on-the-ground testing regimen in Burkina Faso. Currently, the team is testing the spores on mosquitoes contained in a custom-built enclosure that resembles a greenhouse, with walls made of netting instead of glass.
“This is our first in-depth study of the effects toxin-expressing fungi have on mosquitoes, beyond their ability to kill faster,” Lovett concluded. “This is also our broadest characterization of our arsenal of insect-killing spider and scorpion toxins. Our results directly extend our understanding of how these technologies may be used in the field against mosquito pests.”