July 1, 2010 (Vol. 30, No. 13)
ZFN Technology Is Being Used to Facilitate the Genetic Engineering of Mammalian Cells
For decades, mammalian genomes have remained resistant to targeted modification and researchers have generally lacked robust tools for efficient and routine mutagenesis. This is not surprising as mammalian genomes are littered with large amounts of repetitive elements, some of which constitute ~21% of the entire genome.
If the human genome were more permissive to homologous recombination, the stability of the genome would be largely compromised. Thus, in light of this and other protective mechanisms mammalian cells have established for genome stability, the genetic engineering of mammalian cells has remained difficult.
In cell types where homologous recombination is naturally suppressed, methods have been developed to induce hyper-recombinant phenotypes. For example, phage proteins have been used to globally increase the rate of homologous recombination in Escherichia coli and Mycobacterium tuberculosis.
This technological advancement facilitated the rapid generation of a complete, genome-wide knockout library of E. coli strains, each lacking a specific gene. However, application of this or similar technologies is impractical in mammalian cells since a global increase in recombination would destabilize the genome through random recombination of repetitive elements. Indeed, such genomic instability is known to be associated with many human diseases.
The recent development of engineered zinc finger nucleases (ZFNs) provides researchers with a technological solution to mammalian genome editing. ZFNs can be designed to generate site-specific double-strand breaks (DSBs), which are repaired by either nonhomologous end joining (NHEJ), leading to gene knockout or homologous recombination (HR), achieving precise gene replacement, insertion, or deletion (Figure 1).
In this way, gene editing becomes possible in a localized area without compromising the stability of the entire genome. Consequently, in the area surrounding a ZFN-mediated DSB, the rate of DNA repair can be elevated by several orders of magnitude, often increasing mutagenic frequencies from 10-6–10-5 up to 10-2 without global changes in genomic stability.
Such a large increase in efficiency has enabled the direct screening of clonal cells for mutations in convenient 96-well formats, without relying on antibiotic selection markers. The freedom from using antibiotic selection enables a new level of “surgical” genome modification such as the mutation of codons and the modification of single nucleotide polymorphisms (SNPs).
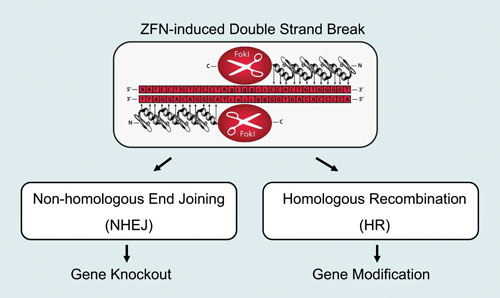
Figure 1. A zinc finger nuclease (ZFN) is composed of two functional domains: (A) an engineered zinc finger protein that binds the target DNA and (B) the type II restriction endonuclease FokI.
Making Delicate Point Mutations
In the decades since the development of recombinant DNA technology, the exploration of gene function has largely relied on creating the desired mutations to protein coding sequences as cDNA harbored on exogenous plasmids. The modified genes are then shuttled into human cells and expressed from exogenous promoters.
Endogenous gene expression, however, occurs in a much more complex environment, reliant on exon-intron splicing and many other gene regulation factors mediated by interactions of 5´-UTR and 3´-UTR regions with control elements such as transcription factors and microRNAs. Additionally, the sheer size of many genes (20–100 kb and higher) makes it cumbersome to isolate and express them on exogenous plasmids with their exon, intron, and UTR structures intact.
The creation of targeted point mutations at specific positions on the mammalian chromosome has proven to be a difficult task, even when homologous recombination rates are elevated in specific cell types. ZFNs enable delicate, specific mutagenesis of single base sites in the native context of the gene, lending more biological relevance to the resulting data generated by targeted mutagenesis.
The ability to make such mutations enables a more rigorous investigation of SNPs, in which single base pair differences are associated with disease states. ZFN-mediated single base pair mutations have been accomplished at several different genomic loci using ZFNs in conjunction with a donor DNA containing the desired mutation.
Transgenic Animals
In the last 20 years, the availability of knockout mouse technology has established the mouse as the most widely used model system in biomedical research. The key enabling aspect of knockout mouse technology lies in the ease with which mouse embryonic stem (mES) cells can be cultured, genetically modified, cloned, and implanted into blastocysts to derive chimeric animals.
In particular, mES cells are relatively amenable to targeted genetic modification using HR and selectable markers while other cell types are not. For instance, large efforts have been mounted to establish a similar knockout rat approach by focusing on the culture of rat ES cells. However, while this effort culminated in the successful isolation of rat ES cells in 2008, gene-targeting techniques have not been developed, underlining the need for alternative genome manipulation techniques.
In 2009, the first targeted knockout rats were created via microinjection of ZFNs into single-cell embryos, resulting from a collaboration involving Sigma-Aldrich, Sangamo BioSciences, the Medical College of Wisconsin, OMT, and INSERM. The gene knockout process relied on the ability of ZFNs to create high frequency insertions and deletions through the DNA repair process of NHEJ.
The method for delivering ZFNs as capped poly-tailed mRNA via embryo microinjection and screening of subsequent animals was rapid and efficient, enabling the production of founders in as little as two months post-microinjection by screening less than 100 animals. Independent follow-up efforts have produced IL2Rγ knockout rats.
Most importantly, the method by which the ZFN-derived knockout rat was generated suggests a general solution to targeted gene knockout in other species. ZFN-mediated gene knockout does not require the establishment of ES cell culture for the species of interest, but simply requires that embryo isolation, injection, and implantation protocols be well characterized.
Many animal species have embryo-manipulation methods robust enough to support the creation of transgenic animals, including mice, rats, rabbits, chickens, sheep, goats, cows, and pigs. The same methods used to create transgenic animals can be used to efficiently deliver ZFNs to embryos and potentially derive knockout animals. Even when ES cell culture is established for a given species, ZFNs offer a faster route to modified animals by manipulating the embryo directly without the need for assembling targeting plasmids required for gene knockout in ES cells (Figure 2).
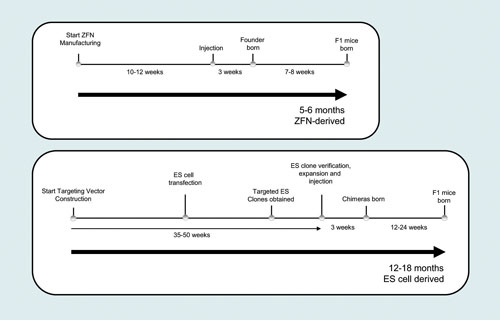
Figure 2. ZFNs reduce the timeline to create knockout mice by eliminating the need for construction of targeting vectors and gene targeting of embryonic stem (ES) cells. Following ZFN manufacturing, mRNA encoding the ZFN is injected into embryos where they produce NHEJ-derived deletions and insertions that disrupt gene function. The ZFN-induced mutations are frequently incorporated into the germline, reducing the required breeding time and effort to derive homozygous knockout animals.
Conclusion
ZFNs have enabled a robust process to target editing of the genome despite the natural mechanisms developed by mammalian genomes to prevent genome instability. While this article has highlighted two major ZFN applications, point mutagenesis and transgenics, ZFNs have also broken new ground in creating isogenic knockout human cell lines, even in cases of aneuploid karyotypes common in many cancer cells.
Additionally, many examples now exist in the literature illustrating the utility of ZFNs to deliver reporter genes to specific locations, reporting the expression of genes with all native transcriptional and translational control elements intact. Together, these various new modes of ZFN-driven mammalian genome editing have changed the questions that scientists can ask and are being rapidly expanded into a host of new cell types and animal models.
Gregory D. Davis, Ph.D. ([email protected]), is principal R&D scientist, ZFN Group, Sigma-Aldrich, and Xiaoxia Cui, Ph.D., is principal R&D scientist, Sigma Advanced Genetic Engineering (SAGE Labs), Sigma- Aldrich.



