August 1, 2009 (Vol. 29, No. 14)
Identifying Markers Is Not Enough, Researchers Must Understand Their Attributes As Well
The role of biomarkers in making personalized medicine a reality is captivating researchers despite development, clinical validation, and regulatory challenges. A recent BCC Research report estimates that the global biomarker market will reach $12.8 billion by 2012. Many companies and organizations covet a piece of this market and are actively seeking to streamline biomarker research. Two upcoming conferences—IBC’s “New Frontiers in Cancer Drug Development” and CHI’s “Accelerating Development & Advancing Personalized Therapy”—will provide forums for discussions about ongoing biomarker research efforts.
A focus on signaling pathways has provided Curis with a novel approach to drug discovery. “There’s a lot of redundancy built into these pathways, so if you have a highly specific blockade, you may get an initial response, but the majority of time tumors can adapt and bypass it. So our scientists focus on how to more effectively block the signaling and be able to knock out a network instead of a distinct node of intervention,” explains Daniel Passeri, president and CEO.
More specifically, Passeri says, preclinical data has shown that if one uses a deacetylase inhibitor with a kinase inhibitor, there’s a synergistic intervention to knock out key networks. His research group found they could build a single drug scaffold with an active kinase moiety that specifically binds to EGFR and includes an HDAC inhibitor.
Passeri says that there are potentially several competitive attributes to this approach that have yet to be seen in the clinic, though there are early promising clinical results. One of the challenges in combination therapy is striving to hit two or three distinct targets within a patient population with different chemical entities. “The pharmacokinetics of these drugs are typically distinct so the drugs are degraded at different rates, and they may also have different pharmacodynamics, which may affect patient compliance.”
The company’s recently patented drug, CUDC-101, hits multiple targets in cancer cells, including HDAC, EGFR, and HER2. It has aligned pharmacokinetics so the drug is degraded in a coordinated manner and provides a better toxicity profile since more of it is concentrated in the tumor, Passeri reports. This novel agent also has a potency enhancement of approximately five- to tenfold over the prototype-approved HDAC inhibitor, he adds.
Since it is administered intravenously, it bypasses the gastrointestinal tract and enables higher dosing levels. “The key advantage here is that we’re able to achieve network disruption with a single agent, which we believe will be more efficacious and better tolerated, and also provide a significant cost advantage since it’s a single drug.” CUDC-101 is currently in Phase I dose-escalation studies. Next in development is an HDAC p13-kinase inhibitor.
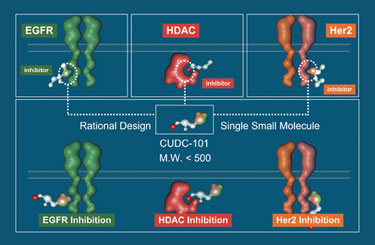
Curis’ CUDC-101 potently and selectively binds to three key cancer targets (EGFR, HDAC, and HER2).
Individualized Cancer Treatment
Genstruct is applying the concepts of artificial intelligence to develop a three-tier architecture for determining biological mechanisms, in an attempt to increase the speed and success of drug discovery and development. Knowledge Assembly® is based on the company’s large generic knowledge base, which can be customized and augmented to specifically represent each cell type, tissue, or organ system, explains Keith Elliston, Ph.D., president and CEO.
“We’ll take that information with actual experimental data and run it through reverse causal analysis to find what caused that data to be produced; it represents biology that has changed with a perturbation like disease or toxicity. This is a subset we call the Causal Network Model—the cause-and-effect reactions that occurred, to change from a normal state to a disease state,” Dr. Elliston says.
This approach overcomes some of the key challenges of designing biomarkers. “Our system allows us to take different data, like gene-expression data, to build a model and design the biomarker, this can be a protein, an imaging reagent, anything the model tells me about the biology of the system. I then know why that is a marker for a specific mechanism because I have a complete mechanistic model of how that particular function works.” Furthermore, he adds, the mechanistic model is 20 times more efficient than a direct empirical method.
Models of patients can be compared to map out the entire extent of functional variation between individuals for disease state and drug response. This is especially pertinent for modeling individual tumors to define dysregulated pathways and guide therapy.
MyPath™ builds a network for a patient’s disease state to help match the patient to the best therapies. Dr. Elliston says the company has begun implementing this assay in a clinical cancer setting, and it works well with both solid and hematological cancers. “We think of it as individualized medicine. Our models can, with a small assay, define the entire state of the patient’s disease and then match that to the therapies right for that patient’s biology.”
Effective Animal Models
Instead of focusing on a particular platform technology, Synta Pharmaceuticals’ biomarker approach zeros in on important questions in the biology of human disease. “We let the biology choose the assay, not the other way around,” comments Kevin Foley, Ph.D., director of in vivo pharmacology.
Synta’s scientists try to understand the molecular basis of a disease and develop animal models to test compounds for the specific biology that translates into human disease. “Traditionally, the problem has been the gold-standard model. If you’re developing a drug for arthritis, you use the collagen-induced arthritis model—everyone uses that model no matter what type of drug is being developed. That’s where you run into problems—the gold-standard model reflects some of the biology going on but not all of it. What we try to do is link animal models and human disease to have a better understanding of both ends so you can choose the best development pathway to develop drugs.”
These animal models have proven effective in development of the company’s vascular disrupting agent, STA-9584, which targets tumor vasculature. It binds to the colchicine site of tubulin, selectively targeting endothelial cells of tumors, but not normal tissue. This shuts off blood flow in the entire tumor (not just the center), leading to hypoxia and necrosis.
In preclinical animal models, the agent has shown improved therapeutic index relative to other VDAs such as combretastatin, Dr. Foley says. Necrosis and apoptosis will be used as biomarkers in the clinic to examine what happens in the tumors when treated with STA-9584. “We’re not using animal models to say, ‘yes, this drug is going to be clinically active,’ but we’re using it to say ‘it’s more likely to be clinically active compared to competing agents in this field.’”
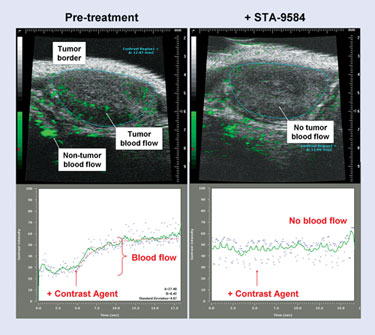
According to Synta Pharmaceuticals, STA-9584 completely shuts off tumor blood flow in a mouse breast carcinoma tumor model.
Modeling and Simulation
In silico disease models and virtual patients are the basis of Entelos’ PhysioLab® platforms, which are used to identify and validate drug targets and develop biomarkers.
“Identifying a biomarker means identifying a selection of measurable attributes that would provide the desired result,” says Alex Bangs, Ph.D., cofounder and CTO. This is done by running simulations of many patients under the clinical conditions of interest on current standards of care, new therapeutic options, competitor products, etc., and then mining those simulation results to identify the best marker panel. “Having a model that includes more physiology of the biology around the disease represented gives us a rich environment to mine for these markers. Once we have identified the panel of markers, we can then trace them back into the physiology and understand why they are good markers and have confidence in using them to distinguish patients.”
Dr. Bangs emphasizes that the uniqueness of the PhysioLab platform is that it’s a mechanistic model, versus a statistical model that cannot distinguish relationships between high-level clinical markers. “Having the deeper representation of physiology (the mechanisms) means there are more potential places to identify markers in the system that could be predictive. To do the same in a clinical trial, you would have to measure too many things because you may not know in advance which would be most predictive.”
It’s important to simulate whole populations when looking at biomarkers to get a good idea of how patients will respond before the drug goes to market. “We create many virtual patients by varying genetic and environmental conditions to statistically mirror real clinical populations through public studies or previous clinical trials. The data is used to help us create the appropriate population.”
The company currently has PhysioLab platforms for asthma, type I and II diabetes, rheumatoid arthritis, cholesterol, and metabolism, and is developing cardiovascular and skin allergy platforms. Dr. Bangs says the FDA is reviewing mechanistic models in terms of how they can be helpful for questions regarding patient variability and for new therapeutics where there isn’t an existing body of clinical data.
Systems Approach
“A single marker or molecule does not control what the cell does—you need to look at the pathway. That’s why a systems approach is necessary—you have to understand the functional aspects so you can find biomarkers and look at them in a coordinated manner, rather than one at a time,” states Gordon Mills, M.D., Ph.D., chair of the department of systems biology, and codirector of the Kleberg Center for Molecular Markers at M.D. Anderson Cancer Center.
Dr. Mills says there are currently several companies that won’t build a drug without a proposed biomarker. “That’s as big a paradigm shift as you can imagine from when companies were worried about segmentation of the market to currently where they are worried about finding a drug that works.”
Dr. Mills’ research group is performing mutational detection across large numbers of individuals to identify genetic aberrations to match patients to particular therapies. A new pilot project, called the “Kleberg Program in Personalized Medicine” will involve 10,000 patients. “We will perform mutation detection in patients for whom there is no standard therapy, and who are likely to be entered into clinical trials. The idea will be to use that data to choose the best clinical trial for them independent of their disease of origin.”
The mutation detection assay will be done on the patient’s original diagnostic paraffin block, which has been designed to be CLIA-compatible and has been validated. Dr. Mills says the idea is to target the patient for treatment with an appropriate drug and determine in the trial whether targeting, meaning the drug fits the mutation versus standard of care, will improve outcome. “This pilot project is really to determine if mutational detection for a specific type of biomarker can be used.”
Oncology Biomarker Development
Eli Lilly’s corporate strategy of tailored therapeutics is focused not only on improving outcomes for patients, but to “assure the right drug for the right patient at the right time and dose,” says John Bloom, DVM, Ph.D., executive director, diagnostic and experimental medicine. “Our strategy is to leverage biomarkers to achieve two high-level things: defining probability of technical success so that we’re able to apply quick wins and quick fails, and differentiating our products in the market.”
Dr. Bloom adds that developing oncology therapeutics has its own requirements. These include evidence of target engagement, defined entities the drug is directed toward and the dose-response relationship, and determination of the biologically effective dose and markers to illustrate MOA. There is also the challenge of the normal biology of the host versus the biology of the tumor.
Currently, there are well-characterized pathways of metastases, apoptosis, and DNA repair. “Using molecular profiling, we can better anticipate markers for pathway modulation. Platforms and tools such as mutation analysis or translocations are providing opportunities to advance science at a pace that’s faster than drug development, which is a problem.”
The challenges of applying these tools to oncology include having the right surrogate to validate targets in early discovery. “Cell lines and xenografts are remote with limited relevance to cancer in the human body. Access to tumor and relevant tissue is a major problem—we have to rely on solid tissue markers and use immunohistochemistry, which is arduous and expensive.” He says that the company is building a capability to integrate the steps of translational biomarker research. “This remains a major unmet need in the area of cancer biomarker development in an R&D setting.”
In addition, Dr. Bloom says that the tumor’s phenotype and genotype often change over treatment, so establishing markers to track these changes can be difficult. Cell-free DNA and circulating tumor cells may provide a noninvasive way to monitor tumor cell biology.
“Blockbuster drugs don’t work in every patient. There are ways to stratify patients,” summarizes Dr. Bloom. “It’s not only going to be good business, but we’re going to be compelled to do it.”



