November 1, 2012 (Vol. 32, No. 19)
Shotgun Mass Spectrometry Can Be Used to Improve Production QC
Before a biopharmaceutical company releases a new batch of biotherapeutics, it needs to conduct comparability studies with FDA-approved production lots to demonstrate the new batch is indistinguishable from officially approved lots. While such quality assessment processes are well-established for small molecule drugs, the process presents a new challenge for manufacturers of biotherapeutics.
Mass spectrometry seems to be the technology platform of choice when comparing newly produced batches of biotherapeutics with previously approved reference samples. While detailed regulations for the quality assessment of biotherapeutics are still in development, an increasing number of biopharmaceutical companies (including producers of biosimilars) have adopted mass spectrometry-based workflows in their quality control processes for biotherapeutics.
Obviously, the quality assessment process needs to be reliable and efficient. An important success factor is hereby the standardization and automation of the data management and analysis process. It requires a comprehensive and scalable data-analysis platform such as that available with Genedata Expressionist for Mass Spectrometry.
This tutorial will outline a data-analysis process for assessing the quality of biologics drugs using Genedata Expressionist for Mass Spectrometry.
Over the last decade mass spectrometry (MS) has become the preeminent method for the quantitative analysis of proteins. While characterization of intact proteins is possible using modern high-resolution MS instruments, most applications rely on a tryptic digest of the complete protein to smaller peptides followed by a liquid chromatographic separation and subsequent mass spectrometry of the peptide mixtures (LC-MS).
As there is a positive correlation between the abundance of peptide in a given sample and the magnitude of the corresponding MS signals, MS data is very useful in many life science applications ranging from patient stratification, precision medicine, and predictive toxicology in drug development, to microbial strain optimization in industrial biotechnology.
MS data analysis requires the quantitation and identification of peptide peaks across samples followed by multivariate statistical analysis to find significant peptide and protein biomarkers. Including post-translational modifications, the human proteome is estimated to contain more than 100,000 distinct proteins. Typical LC-MS experiments of human plasma and serum can reliably detect and measure about 25,000 peptide fragments from about 5,000 proteins. As such, protein biomarker discovery truly is a search for a needle in a haystack.
Biotherapeutics are produced by genetically modified organisms (expression systems) in industrial-scale fermentors followed by harvesting, purification, and filling. Some of the factors that can negatively impact production and result in product deviations from the original protein (i.e., changes in safety and efficacy profiles of the final protein) include:
- Potential mutations of the expression system
- Known and unknown viral contaminants
- Post-translational modifications
Before releasing a new biotherapeutics batch, manufacturers must verify that the product matches the reference drug originally approved. While this process is straightforward for small molecule drugs, rigorous establishment of protein bioequivalency is significantly more challenging.
Following the successful adoption of label-free MS methods in different protein analytics applications, manufacturers of protein drugs are now utilizing label-free MS methods to establish bioequivalence: post-production samples of purified proteins are obtained from the production line and are subjected to an enzymatic digestion. The resulting mixture of small peptides is injected into a liquid-chromatography column that is coupled to a high-resolution MS instrument (LC-MS). Alternatively, the instrument can be set to acquire peptide fragmentation data for peak identification using protein databases (LC-MS/MS).
Each peak in the resulting MS chromatogram corresponds to a single peptide, which corresponds to a small sequence of the biotherapeutic protein (Figure 1). By performing a peak detection and raw data quantitation, one can effectively measure the amount of a given peptide present in the protein.
In turn, these numbers can be compared to those obtained from the reference samples and any statistically significant differences between the two samples are indicative of modifications to the protein drug that may give cause for concern and warrant further investigation before release.
While straightforward in theory, to successfully work within a production environment this approach requires:
- Carefully controlled experimental conditions
- Scalable workflow to process sufficient technical replicates
- Standardized and automated data analysis process to produce reliable results
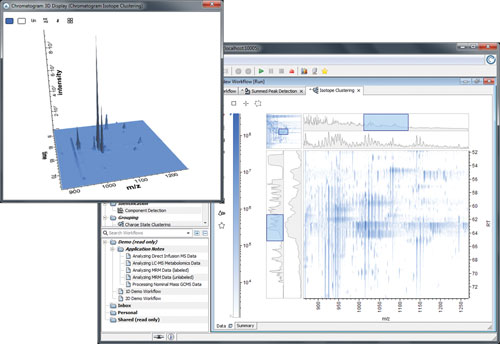
Figure 1. LC-MS chromatogram from a single sample with more than a billion data points.
Quality-Assessment Software
Genedata Expressionist for Mass Spectrometry enables scientists to implement an end-to-end data-analysis workflow from raw MS instrument readouts to auditable quality reports. With support for modern MS instruments from Thermo (LTQ Orbitrap®), Waters (Xevo® and Synapt®), and AB Sciex (QTRAP®), users can adapt data analysis to meet their specific reporting standards.
Using Genedata, researchers can leverage capabilities such as:
- Real-time monitoring: Real-time monitoring of results and live adjustments to processing rules enables users to quickly address quality issues identified using interactive data exploration and flexible analysis workflows.
- Scalability: The number of biotherapeutics to be tested as well as the number of replicate measurements to be taken is rapidly increasing, and therefore the software must process, analyze, and visualize huge quantities of experimental results.
- Instrument independence: Most MS laboratories operate multiple instruments from different vendors. The flexible workflow system enables users to setup processing rules addressing different instrument performance characteristics and provides a common interface to all MS instruments.
Case Study
The following example illustrates the successful application of Genedata Expressionist for MS for the quality assessment of biotherapeutics production in a leading biopharmaceutical company.
Production lot/batch samples are analyzed using a tryptic digestion followed by LC-MS and compared with the FDA-approved biotherapeutic batch. Before employing Genedata Expressionist, data analysis was done in spreadsheets, included multiple file conversion steps, and a variety of nonstandardized processing steps. This created a data-analysis bottleneck, which prevented the process to scale up and reach production-scale targets. In addition, the lack of technical replicates and inability to easily increase throughput resulted in many false positive calls, shipment delays, and significant loss of revenues.
Genedata Expressionist for Mass Spectrometry was introduced to address these issues. It provides a workflow system supporting automation and repeatable data processing with a highly scalable architecture that enables real-time analysis of large experiments. Together with a customized data export, the built-in support for all major MS instrument vendors allowed the team to eliminate manual file conversions and implement an auditable end-to-end solution that supports in-house validation and regulatory filings (Figure 2).
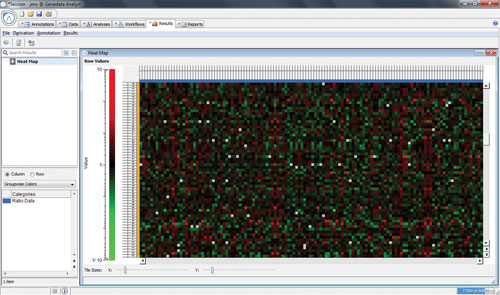
Figure 2. Heat map comparing production batches to reference samples with very little change in peptide abundances.
Outlook
Biotherapeutics will continue to play an increasingly important role in the treatment of a wide range of relevant diseases. This expansion is increasing the number of biotherapeutics companies and contract manufacturing organizations capitalizing on the advantages of an integrated MS data analysis platform for quality control. Comprehensive and automated software solutions such as Genedata Expressionist for Mass Spectrometry help to improve productivity and process scalability by enabling better experimental designs.
Finally, with patents of the first group of biologics drugs about to expire, regulatory agencies are working on establishing policies governing the approval of biosimilars. While these policies are evolving, it is reasonable to assume that the MS methods described here are going to play in important role in future regulatory frameworks.
Jens Hoefkens, Ph.D. ([email protected]), leads the Genedata Expressionist business unit at Genedata.



