September 1, 2011 (Vol. 31, No. 15)
Optimization of Key Parameters Essential for Robust Protein Production
Biotherapeutic protein production in mammalian cell systems is becoming commonplace as drug targets increase in complexity. Proper protein folding, assembly, and post-translational modifications are vital to a fully bioreactive end-product, bringing mammalian cell expression systems that closely mimic human processing to the forefront.
Suspension cell lines derived from CHO and HEK 293 cells are commonly used for mammalian protein production. These cell lines have many desirable traits including high expression levels, scalability (density and volume), and especially in the case of CHO cells, a history of regulatory approval.
Clinical biotherapeutics are frequently generated using stable transfectants for batch-to-batch consistency and low cost at extremely large scale. Many advances spanning the last decade such as improved cell lines, expression vectors, culture medium, and delivery methods have led to the adoption of transient transfection methods for mammalian protein expression.
In many drug discovery applications, it is beneficial to screen protein constructs quickly using transient transfection methods, allowing for the evaluation of various target molecules or protein isoforms simultaneously. In many instances, transient transfections are performed in parallel while more resource-intensive stable cell lines are under development.
Recent advances in transfection technologies, which include the TransIT-PRO® Transfection Kit by Mirus Bio, have allowed researchers to obtain high protein titers in suspension CHO and 293 derived cells in a simple and reproducible manner. Using Mirus Bio’s technology, transfection complexes are prepared in serum-free media by adding plasmid DNA, TransIT-PRO Transfection Reagent, and PRO Boost Reagent.
The PRO Boost Reagent is an optional component and enhances gene expression in certain growth media formulations. After incubating complexes for 10–30 minutes, they can be added directly to cells in normal growth media. Transfection using the TransIT-PRO Transfection Kit eliminates the need for a culture medium change post-transfection and is suitable for both transient and stable transfection. For secreted antibody constructs, optimal titers are typically obtained 5–7 days post-transfection in batch fermentation.
Several parameters should be considered during the optimization of transient transfection protocols including cell density at the time of transfection, DNA concentration, reagent-to-DNA ratio, and cell culture medium.
Cell Density
Cellular health is an important consideration prior to any tissue culture experiment. Ideally, cells should have a consistent doubling time and high viability. Contamination with bacteria, yeast, virus, or mycoplasma will not only impair cellular health but also transfection efficiencies.
For suspension cells that can grow to very high densities, it is important to optimize the cell density at the time of transfection. Efficiencies are highest when cells are actively dividing during transfection, which assists in the nuclear entry of the plasmid DNA. Figure 1 shows a titration of CHO suspension cell densities ranging from 0.25 x 106–2.0 x 106 cells/mL. The maximal number of GFP-expressing cells (approximately 60%) is detected at cell densities between 0.5 x 106–1.0 x 106 cells/mL at the time of transfection.
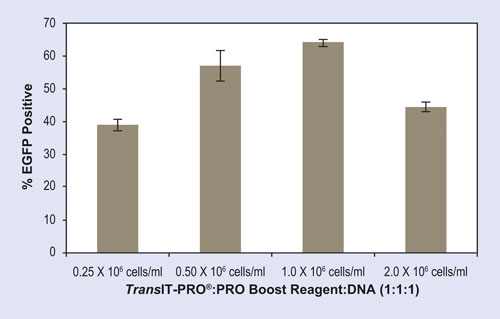
Figure 1. Optimal cell density at the time of transfection is 0.5 x 106–1.0 x 106 cells/mL. CHO-S cells were transfected using the TransIT-PRO Transfection Kit at a range of cell densities at the time of transfection. Complexes were formed using a 1:1:1 ratio of TransIT-PRO:Boost Reagent:DNA using an enhanced green fluorescence protein (EGFP) reporter plasmid. GFP transfection efficiency was determined at 48 hours post-transfection using flow cytometry. Transfections were performed in 24-well deep-well shaker blocks using FreeStyle™ CHO-S cells cultured in FreeStyle CHO Expression media (2 mL/well). Error bars represent the standard deviation of triplicate wells.
DNA Concentration
The plasmid DNA concentration needs to be sufficient to obtain high gene expression. However, extremely high DNA levels can be toxic and/or cost-prohibitive. Figure 2 examines a range of plasmid DNA concentrations from 0.25–3.0 µg per mL of culture using three different doses of TransIT-PRO and PRO Boost Reagent. Typically, 1.0–1.5 µg DNA per mL of culture is the optimal concentration for maximal expression. In some cell types, increased plasmid DNA concentration results in improved transfection efficiencies, therefore the benefit of increased expression needs to be weighed against the added cost.
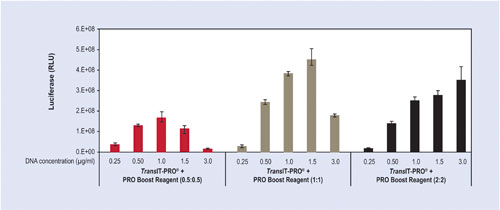
Figure 2. Titration of DNA concentration and reagent-to-DNA ratio using the TransIT-PRO Transfection Kit. Luciferase protein expression was compared at varying plasmid concentrations (0.25–3.0 µg/mL) and using three different ratios of TransIT-PRO:PRO Boost Reagent (0.5:0.5, 1:1, and 2:2) per µg of DNA. Transfections were performed in 24-well deep-well shaker blocks using FreeStyle CHO-S cells cultured in BD Select CD1000 media (2 mL/well). Cells were plated at a density of 0.5 x 106 cells/mL, harvested 48 hours post-transfection, and assayed using conventional luciferase assays. Error bars represent the standard deviation of triplicate wells.
Reagent Concentration
Reagent-to-DNA ratio is arguably the most influential parameter in transient transfection. Insufficient or excess reagent quantities can impede DNA delivery. The electrostatic interactions between the cationic transfection reagent and negatively charged plasmid DNA facilitate the plasma membrane binding and internalization of the transfection complex. However, excess positive charge is typically associated with cellular toxicity and necessitates a titration of transfection reagent-to-DNA ratios to determine peak protein expression.
Figure 2 compares three different levels of TransIT-PRO and PRO Boost Reagent (0.5 µL–2.0 µL per µg of DNA); the highest luciferase expression is obtained with TransIT-PRO and PRO Boost Reagents at a concentration of 1.0 µL each per 1.0 µg of DNA.
Media Formulation
Transfection efficiencies are significantly influenced by the culture growth media, and there are many commercially available serum-free complete growth media for biotherapeutic protein production. Since these media formulations are proprietary, it may be necessary to test several media formulations to find one that is complementary with the desired transfection method.
Figure 3 illustrates suspension CHO cells that have been adapted to five different commercially available media formulations. Cells adapted to the respective media were transfected with a plasmid encoding a human IgG1 antibody construct using multiple transfection methods including: TransIT-PRO Transfection Kit, Reagent P, and Reagent F (Figure 3).
Secreted antibody titers varied by greater than 10-fold, depending on the media type, within a given transfection methodology. Additionally, not all transfection technologies exhibited broad-spectrum media compatibility. The highest antibody titers were obtained when transfections were performed with the TransIT-PRO Transfection Kit in combination with BD Select™ CD 1000 medium (BD Biosciences).
High-efficiency transient transfection of suspension CHO-derived cells enables efficient manufacture of complex biotherapeutics. Notably, protein yield is highly dependent on the intrinsic properties of the recombinant protein; two antibody constructs of the same subtype can produce vastly different protein titers. When comparing two or more transfection methods, perform transfections on the same day with the same plasmid DNA construct and the same batch of cells. This allows cellular and experimental variables to be minimized.
New transfection methods such as the TransIT-PRO Transfection Kit allow researchers to obtain higher protein titers in a straightforward and consistent manner. Further optimization of key transfection parameters enables researchers to obtain high transfection efficiencies with sufficient protein yields to accelerate drug discovery.
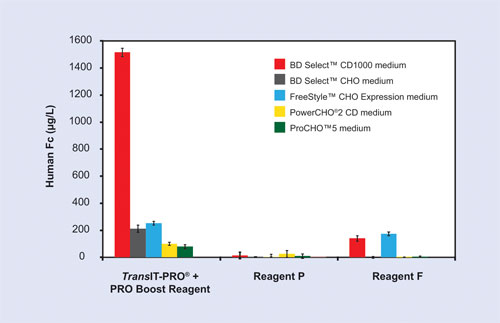
Figure 3. Protein yield is dependent on media formulation and transfection method. FreeStyle CHO-S cells were adapted to five representative growth media. Cells were transfected using the TransIT-PRO and PRO Boost Reagent (1:1:1), Reagent P (4:1), or Reagent F (1:1) transfection reagents. Transfections were performed in 24-well deep well shaker blocks using 1 µg DNA per milliliter of culture and 0.5 x 106 cells/mL at the time of transfection. Human IgG1 was quantitated from day 5 clarified supernatants and analyzed by a standard sandwich ELISA. Error bars represent the standard deviation of triplicate wells.
Laura Juckem, Ph.D. ([email protected]), is R&D group leader at Mirus Bio.



