October 1, 2011 (Vol. 31, No. 17)
James M. Wilson, M.D., Ph.D.
A Scientist’s View from the Front Lines of Basic and Applied Clinical Research
The potential of treating or preventing disease by modifying the expression of one’s genes has captured the imagination of the scientific community ever since the discovery of DNA.
This therapeutic concept, called gene therapy, emerged as a very literal clinical application of modern day molecular biology. While the concept may seem simple, its translation into effective therapies has been challenging. After three decades of scientific development and many failed clinical trials, the field of gene therapy is emerging as a viable approach for treating a broad spectrum of acquired and inherited diseases.
The events that led up to this impending revolution are summarized below.

James M. Wilson, M.D., Ph.D.
Birth of Gene Therapy
The initial concept of gene therapy was to introduce into the cells of a patient a therapeutic gene in an approach called gene replacement. This intervention simply added an additional gene without directly perturbing the structure of an endogenous nonfunctioning or pathogenic gene.
The technical challenge was one of drug delivery, in which the drug is DNA expressing a new gene. The challenge was delivering these very large and highly charged molecules to a very specific population of cells in a way that the genes migrate into the nucleus. The concept that made the field of gene therapy an experimental reality was the notion of using viruses as delivery vehicles for therapeutic genes.
The first virus used to develop a vector for gene therapy was from the family of murine retroviruses. It was possible to create replication-defective versions of these viruses that could be engineered to contain therapeutic transgenes. The transfer and expression of these transgenes, in a process called transduction, was impressively high if the cells were replicating in culture when exposed to the vector. The vector genome persisted in the cells and their progeny within chromosomes as randomly integrated proviruses.
The initial clinical applications of gene therapy focused on an approach called ex vivo gene therapy in which cells from the patient are harvested and cultivated with retroviral vectors in vitro prior to re-implantation into the person from which they were derived. The ex vivo approach, which made the most sense to pursue as a proof-of-concept, was based on the transplantation of genetically modified autologous bone marrow-derived hematopoietic stem cells (HSCs).
Target diseases were genetic in nature and involved the hematopoietic lineages, including erythroid-based diseases such as sickle cell or thalassemia or lymphoid- based disorders such as severe combined immune deficiency (SCID) caused by a defect in the enzyme adenosine deaminase (ADA). Experiments in mice confirmed the feasibility of this approach, although demonstrating transduction of human HSCs ex vivo proved to be difficult.
The first human applications of gene therapy focused on ex vivo gene therapy using autologous lymphocytes, which were easier to transduce and characterize than HSCs. The problem with this approach is that lymphocytes were not capable of self renewal so the genetic graft would be temporary. Nevertheless, clinical trials proceeded with ADA deficiency using lymphocytes as targets although immune reconstitution was incomplete and transient.1
The real importance of this trial was not its outcome but rather the fact that it signaled the birth of the field of gene therapy.
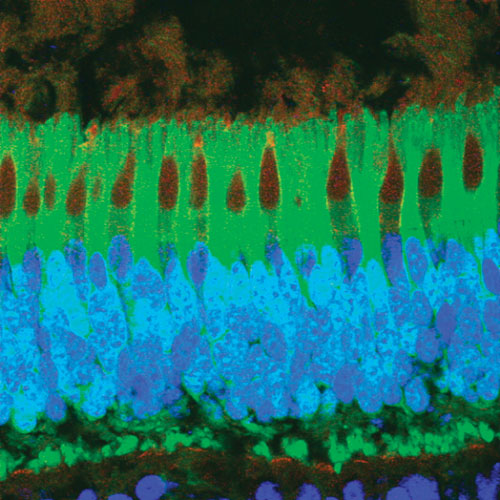
AAV8 has shown substantially higher transduction in a number of highly relevant target cells such as liver, muscle, and photoreceptors of the retina. A photo-receptor layer of a monkey retina after subretinal injection of AAV8 expressing GFP (green) in rods and cones. Cone photoreceptors have been stained with peanut agglutinin (red) and nuclei with DAPI (blue).
Explosive Growth
The introduction of gene therapy into the clinic ignited a firestorm of interest by many stakeholders, including the scientific community. The application of gene therapy was envisioned for a broad range of disorders. The limiting step was, and still remains, technologies capable of safely and effectively transferring genes into a wider array of target cells beyond those of bone marrow.
Gene transfer in an ex vivo setting was demonstrated using murine retroviruses in a number of relevant target cells of epithelial and endothelial lineages. Quick translation into the clinic was promised with hopes of early success. Desperation often evoked expectations since many diseases being considered for early clinical trials of gene therapy were disabling and lethal with no available treatments.
It became clear, however, that the only viable way of approaching many of these diseases was to directly deliver the gene to the patient rather than via a transplanted cell in an approach called in vivo gene therapy. The disease that surfaced as an early candidate for in vivo gene therapy was cystic fibrosis (CF), which is caused by a defect in a chloride channel expressed in a variety of epithelial cells, the most important being those of the lung.
Gene therapy for CF would require direct delivery of the normal version of the gene into the lung airways. Unfortunately, vectors based on murine retroviruses were not up to the task for in vivo gene therapy. Vectors were made based on a virus, called the adenovirus, that is normally tropic for the lung.
Direct delivery of this vector into the airway of various animal models did efficiently transduce lung epithelial cells, setting the stage for several clinical trials of in vivo gene therapy in patients with CF.2,3,4
Similarly high levels of transduction were observed with adenoviral vectors in other target organs such as liver, heart and brain to name a few. These preclinical successes with the initiation of the CF trials fueled enthusiasm and expectation far in excess of the reality of the science.
The incredible efficiency with which adenoviral vectors transduce genes in vivo was corroborated in many laboratories. However, a disturbing trend of associated inflammatory type toxicities was being observed. The basis for these toxicities was the activation of adaptive and innate immune responses to the vector and the transduced cells.5 Attempts by us and others to circumvent these immune responses by vector engineering or co–administration of immune modulatory drugs were not successful.
Efforts were directed to develop vector systems that may be less immunogenic with the prime contender being those based on adeno-associated viruses (AAV). This obscure family of viruses was discovered in the 1960–1970s as contaminants in laboratory stocks of adenoviruses.6
Of the six different serotypes discovered, AAV serotype 2 was developed as a vector. It has limited cargo capacity (i.e., <4.8 KB) although it has the remarkable ability of transferring genes in vivo with minimal inflammation or activation of T and B cells against the transgene product. The vector genome persists in a nonintegrated form and continues to express the transgene for the life of the target cell.
However, the genome is destabilized if the target cell divides indicating that the optimal targets are those that are post-mitotic and long lived such as neurons, muscle fibers, photoreceptors, and hepatocytes. Despite these favorable properties, vectors based on AAV serotype 2 have had limited success in the clinic due to low transduction efficiencies and the presence of antibodies in humans as a result of natural AAV infections that inhibit the vector.7,8
Problems with the first-generation vector technologies described in this article led to delays and repeated failures. Stakeholders grew impatient and support for the field waned. A number of research programs endured despite the erosion of support for gene therapy, several of which eventually were tested in the clinic with remarkable success.
Studies of gene therapy for SCID that launched the field in the mid 1980s continued for several decades using the ex vivo bone marrow approach with murine retroviruses as the vectors. The key was to find a disease in which the transduced HSC and/or its progeny had a selective advantage over nontransduced cells.
Incremental advances in the biology of HSCs and techniques of retroviral transduction also helped progress these programs. If successful in these kinds of diseases, the therapy would allow complete repopulation of the treated patient with genetically corrected progeny cells without the need to ablate the endogenous marrow with radiation and chemotherapy, which carries substantial risks of mortality.
One form of SCID caused by a defect in a cytokine signaling receptor was the test case. The results were spectacular in that virtually all who were treated regained substantial immune function.9 Unfortunately, many of these patients eventually developed T-cell leukemias due to integration of the vector genome into a site that activated a tumor-promoting gene.10 Most of these leukemias have been treated and the cohort of patients treated with gene therapy clearly is much better off than they would have been without gene therapy.
The development of cancer is a consequence of the biology of murine retroviral vectors and may not be easily overcome, indicating the need for second-generation technology.
The other widely celebrated clinical success using first-generation technology was in the treatment of children with an inherited form of blindness called Leber congenital amaurosis (LCA). The form of this disorder targeted for gene therapy is defective in an enzyme involved in sensing light and is found in a cell located in the back of the retina.
Studies in dogs with this same disease showed reconstitution of sight following injection of an AAV2 vector into the retina.11 Three independent groups demonstrated reconstitution of visual function in LCA patients treated with AAV2-based gene therapy.12,13,14 The remarkable success of this program was due to unwavering persistence of the scientists and a careful selection of the target disease in an application that was able to succeed despite limitations of the technology.
The ability of gene correction to reconstitute function that can be quantitatively measured noninvasively was critical to the early assessment of efficacy in LCA. The localized delivery of a vector at high concentrations and long dwell times in the back of the retina helped overcome the inherent inefficiency of AAV2.
The clinical successes described here set the stage for a remarkable resurgence in support for gene therapy. The question is how can we leverage these successes into a broader array of applications with the goal of delivering commercial products? These seminal clinical trials established the feasibility of gene therapy in humans and helped overcome a variety of political and regulatory barriers that had surfaced.
Second-Generation Vector Technology
Unfortunately, the first-generation vector technologies deployed in these clinical successes still had the limitations described in this article and will be of limited value beyond these test cases. The good news is that the field has been quietly developing the technology of the future, which is now ready for prime time.
One of the most important limitations of vectors based on murine retroviruses was the need for the target cell to be dividing at the time of exposure to the vector for transduction to occur. This complicates ex vivo applications of stem cells, which often persist in a quiescent state. Furthermore, attempts to induce stem cell division to render them susceptible to retrovirus transduction may irreversibly alter their phenotype essentially losing the capacity for self renewal.
Development of vectors based on the family of retroviruses called lentiviruses represented a major advance in overcoming this limitation. Lentiviruses are pathogens capable of infecting non-dividing cells; the most notorious is HIV.
Investigators at the Salk Institute developed replication-defective vectors based on HIV, which demonstrated efficient stable transduction in nondividing cell targets.15
The first clinical application of lentiviral vectors for a genetic disease deployed an ex vivo bone marrow strategy in a lethal pediatric neurologic disease called adrenoleukodystrophy (ALD). The difference between ALD and the previous work with SCID is that there is no selective advantage of genetically corrected HSCs in ALD as was the case with SCID.
Successful reconstitution of the ALD patient with corrected cells would require very high transduction efficiency ex vivo and some form of ablation of the recipient’s marrow to make space for the corrected cells. The pilot human study was a dramatic scientific and clinical success.16 Patients were repopulated with a high frequency of transduced cells in virtually all hematopoietic lineages that stabilized their disease.
The other technological advance was in the development of second-generation AAV vectors for in vivo gene therapy. We hypothesized that natural variation in the AAV capsid that would occur during natural infections may translate into improved vector performance, albeit in a stochastic manner. We sought to identify AAV capsids from viruses that circulate in primates as infectious agents.
The problem was identifying primates with active AAV infections to obtain samples for virus recovery since the known AAV serotypes were isolated from laboratory stocks of adenoviruses; these AAVs could have been passengers from the initial adenovirus isolations or come from tissue culture components such as bovine serum.
Serologic studies in primates, however, showed antibodies against some of the known AAVs suggesting they circulate in these populations. Based on previous in vitro work we speculated that AAV may remain as a latent genome following resolution of the infection as is the case with the Herpes simplex virus.
We tested this hypothesis by analyzing DNA from primate sources using PCR with primers to conserved regions of the known AAV capsids. The results were startling—latent AAV genomes are widely disseminated through many tissues from a wide array of primates, including macaques, great apes, and humans.17,18,19 Sequencing of these genomes demonstrated a marvelous diversity of structure.
Vectors were created from many of these novel capsids and shown in animal models to be substantially improved over vectors based on the previously known AAVs 1–6. For example, AAV8 shows substantially higher transduction in a number of highly relevant target cells such as liver, muscle, and photoreceptors of the retina.
A clinical trial of liver-directed gene transfer with AAV8 in subjects with hemophilia showed partial correction of the clotting defect that has replaced the need for some protein replacement and has been stable.20
AAV9 is a capsid that is capable of transferring its genome across vascular and blood brain barriers and has been shown in preclinical models to treat a variety of cardiac and neurological disorders.21
Another advantage of the novel AAV isolates we recently discovered, such as AAV8 and AAV9 noted above, is that they present diminished problems of host immunity. They show much lower levels of pre-existing immunity in humans which, if present, can diminish efficacy and are less likely to activate destructive T-cell responses.
Next Frontier: Commercialization
The technical challenges of gene therapy have been overcome with over 30 years of investment in basic and translational research. Success in pilot human experiments has been demonstrated with many exciting new applications to emerge.
One of the biggest challenges will be to develop business models to encourage the participation of the biotechnology industry in the development of commercial gene therapy products.
Biotechnology did indeed play a role in the initial development of gene therapy in the 1990s. This occurred in a very different investment climate where promise was as valuable as progress and liquidity could be achieved through IPOs.
These early companies eventually failed with little subsequent investment due to the fact that the field fell out of favor and venture capital investment in the post-dotcom era changed.
However, the positive clinical trials and the upswing in public support have led to a re-engagement of the biopharmaceutical industry. Virtually every major venture capital firm and biopharmaceutical company is in the process of re-examining the value of gene therapy products. Concerns that emerge are not related to technical feasibility but rather to uncertainty about the regulatory process and concerns about the business model.
The fact is that there is no approved gene therapy product in Europe or the U.S.—although this is likely to change in the near future. Challenges regarding the business of gene therapy relate to the point that many of these products will confer long-term effectiveness after a single administration of vector. As one person stated, “How do you price a cure?”
Will the payers be willing to provide sufficient reimbursement for a single curative treatment that is necessary to justify the investment of development? This is further complicated by the fact that many early models of gene therapy involved orphan diseases.
I believe that reimbursement will be adequate so long as the treatment addresses an unmet need in a way that dramatically improves the quality of life of those afflicted with the disease. The field of gene therapy is now ready to achieve this goal.
References
References:
1 Muul, L.M., et al., Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood, 2003. 101(7): p. 2563-9.
2 Crystal, R.G., et al., Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet, 1994. 8(1): p. 42-51.
3 Zabner, J., et al., Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell, 1993. 75(2): p. 207-16.
4 Zuckerman, J.B., et al., A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther, 1999. 10(18): p. 2973-85.
5 Yang, Y., et al., Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol, 1995. 69(4): p. 2004-15.
6 Berns, K.I., Parvoviridae: The Viruses and Their Replication, in Fields Virology 1996.
7 Moss, R.B., et al., Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther, 2007. 18(8): p. 726-32.
8 Manno, C.S., et al., Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med, 2006. 12(3): p. 342-7.
9 Hacein-Bey-Abina, S., et al., Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med, 2002. 346(16): p. 1185-93.
10 Hacein-Bey-Abina, S., et al., LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science, 2003. 302(5644): p. 415-9.
11 Acland, G.M., et al., Gene therapy restores vision in a canine model of childhood blindness. Nat Genet, 2001. 28(1): p. 92-5.
12 Maguire, A.M., et al., Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med, 2008. 358(21): p. 2240-8.
13 Bainbridge, J.W., et al., Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med, 2008. 358(21): p. 2231-9.
14 Cideciyan, A.V., et al., Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther, 2009. 20(9): p. 999-1004.
15 Naldini, L., et al., In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science, 1996. 272(5259): p. 263-7.
16 Cartier, N., et al., Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science, 2009. 326(5954): p. 818-23.
17 Gao, G.P., et al., Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A, 2002. 99(18): p. 11854-9.
18 Gao, G., et al., Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol, 2004. 78(12): p. 6381-8.
19 Gao, G., et al., Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A, 2003. 100(10): p. 6081-6.
20 Ponder, K.P., Hemophilia gene therapy: a Holy Grail found. Mol Ther, 2011. 19(3): p. 427-8.
21 Forsayeth, J.R. and K.S. Bankiewicz, AAV9: Over the Fence and Into the Woods. Mol Ther, 2011. 19(6): p. 1006-7.
James M. Wilson, M.D., Ph.D., ([email protected]) is professor of pathology and laboratory medicine in the gene therapy program at the University of Pennsylvania School of Medicine. He is also editor in chief of Human Gene Therapy, published by Mary Ann Liebert, Inc. Dr. Wilson is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.







