May 1, 2017 (Vol. 37, No. 9)
David Radspinner Ph.D. General Manager GE Healthcare Life Sciences
Creating Flexible Production Capacity Based in Markets All Over the World
Over the past decade, we have witnessed growth in innovative therapies in the biopharmaceutical industry, and an increase in the need for patients across the globe to gain improved access to these biological treatments. Biopharmaceuticals are the world’s fastest-growing class of medicines—of the top 10 therapeutics on the market today, 7 are biopharmaceuticals (2015), and they are about to become even more commonplace with more than 7,000 biologic drug candidate products in research and development. The global market for biopharmaceuticals is estimated at more than $200 billion, and the market is growing around five to six percent annually.
Biosimilars production is also growing rapidly, especially in the emerging economies or in countries where the production of biopharmaceuticals is low. In China, the biosimilars market is expected to grow significantly, reaching approximately $350 million in 2019, up from $44 million in 2009.
There are certain persistent challenges that the industry needs to address before biopharmaceuticals become a more widespread treatment option in the emerging markets. In China, for example, biologics account for only 14% of the medicines prescribed compared to 32% in the U.S., even though China’s population represents 22% of the worldwide cancer incidence, and even though cancer is the leading cause of death in the country. Most biopharmaceutical production is taking place in Europe and in the U.S., and this coincides with a shortage in markets like China, India, and Brazil.
GE Healthcare Life Sciences has spent the past few years addressing that market need and finding alternative ways to quickly establish flexible, multiproduct biomanufacturing facilities in emerging markets that traditionally can take years to build. Our mission was to create a biomanufacturing solution that fits the unique design of a biomanufacturing production process, and not the other way around. We examined the specific manufacturing needs, and we created a facility around the process that was dedicated to optimizing the production of biotherapeutics.
In 2011, GE presented our customers with a flexible, turn-key single-use manufacturing solution that was based on modular facilities and could be built from the ground up in 18 months.
Four central principles guided our design: flexibility, reduced time-to-production, ensured global regulatory compliance, and a known and fixed level of investment from the start. We secured an experienced partner that could help us build a standardized, modular facility that would be easy to put together and could be set up anywhere in the world. This is the KUBio prefabricated facility solution.

Six years ago, GE presented its customers with a flexible, turn-key, single-use manufacturing solution that was based on modular facilities and could be built from the ground up in 18 months.
Modular, Prefabricated Facilities
As the pharmaceutical market developed a rich pipeline of biologics for the treatment of many different diseases, our team saw an unmet need for faster, more turnkey, and more flexible manufacturing capacity in markets all over the world. Our outcome-based approach to enable flexible manufacturing capacity began with our FlexFactory single-use biomanufacturing platform that offered reduced production timelines and increased facility utilization.
Biopharmaceutical manufacturers looking to introduce drug production into areas of the world that had limited access to biologics readily saw the benefits of including single-use technologies into their strategy. However, to establish biomanufacturing in a region with less infrastructure meant building a facility in a greenfield environment, rather than in an existing laboratory or facility like in the U.S. or Europe. Those facilities would need to be constructed faster and with future flexibility in mind.
A modular biomanufacturing approach could incorporate single-use technology to meet evolving needs. Modular facilities reduce time and costs related to pharmaceutical capacity expansion; pave an easier route for pharma companies, both large and small, to enter and grow within the biologic market; and help to bring affordable drugs to markets that have not previously had access to them.
GE’s KUBio effectively provides these capabilities. Essentially, it’s a factory-in-a-box, designed for monoclonal antibody production that is constructed, assembled, fully fitted-out to current good manufacturing practice (cGMP) standards, and ready to run in 18 months. This is significantly faster than traditional factory set-up approaches that typically take up to three years to design and build.
This solution is based on a parallel production path. The modules that make up the facility can be manufactured while the site is prepared and the manufacturing equipment secured. The factory’s heart, the manufacturing line, uses GE’s FlexFactory platform. This means that many elements can be prequalified offsite, saving additional time.
The prefabricated facility arrives in modules that are 80 to 90% pre-equipped; units include heating, ventilation, and air handling systems, cleanrooms, most of the utility equipment, and all other technical installations and piping needed to run the plant. When the modules are assembled into a facility, the actual production platform, the FlexFactory, is installed.
Flexibility is a clear benefit of modular biomanufacturing, with easy integration of new manufacturing unit operations and a design that allows easy expansion by adding further facilities after the initial build.
KUBio facilities with single-use biomanufacturing technologies facilitate multiproduct manufacturing and improve productivity by increasing the number of lots manufactured due to reduced downtime between products. With single-use technologies, it is possible to produce smaller batches, reducing the scale for more precise therapies and keeping abreast of a strong trend that we believe will only accelerate in the future.
In addition, the incorporation of single-use means that these facilities often have a smaller footprint as the equipment required for clean-in-place/steam-in-place (CIP/SIP) is no longer needed, leading to both reduced upfront costs as well as reductions in operational costs on an ongoing basis due to the significant reduction of cleaning and sterilization procedures that are required.
Data shows that KUBio increases manufacturing flexibility and can help reduce build costs up to 50% compared with equivalent traditional brick-and-mortar facility. Additionally, the KUBio facility with a FlexFactory manufacturing line can reduce dioxide emissions by 75% and water and energy usage by approximately 80%.

Modular facilities reduce time and costs related to pharmaceutical capacity expansion and pave an easier route for pharma companies to enter and grow within the biologic market, according to GE Healthcare officials.
Bringing Therapies to Patients
In just a few short years, we have helped the biopharmaceutical industry broaden its impact by helping biopharma companies bring a host of new therapies to emerging markets. KUBio has been quickly recognized as a viable way to produce biologics across the world beyond the U.S. and Europe.
Since the KUBio platform’s inception a few years ago, two KUBio facilities have been initiated. The first one has been opened in China, and the second one will be included in a groundbreaking biotechnology center, also in China. Talks are underway for opportunities in such countries as Brazil, Mexico, South Korea, and Saudi Arabia. There are also numerous FlexFactories all around the world, also outside the growth markets.
This novel solution provides a single point-of-contact throughout the project and beyond including training, validation, and other essential elements for the delivery of biomanufacturing capacity. As biosimilars emerge as a major industry driver for the accessibility and affordability of therapies in many countries, the KUBio and FlexFactory platforms are making it possible to run more batches of varying drugs, as well as speed deployment of manufacturing processes once the biosimilar is approved.
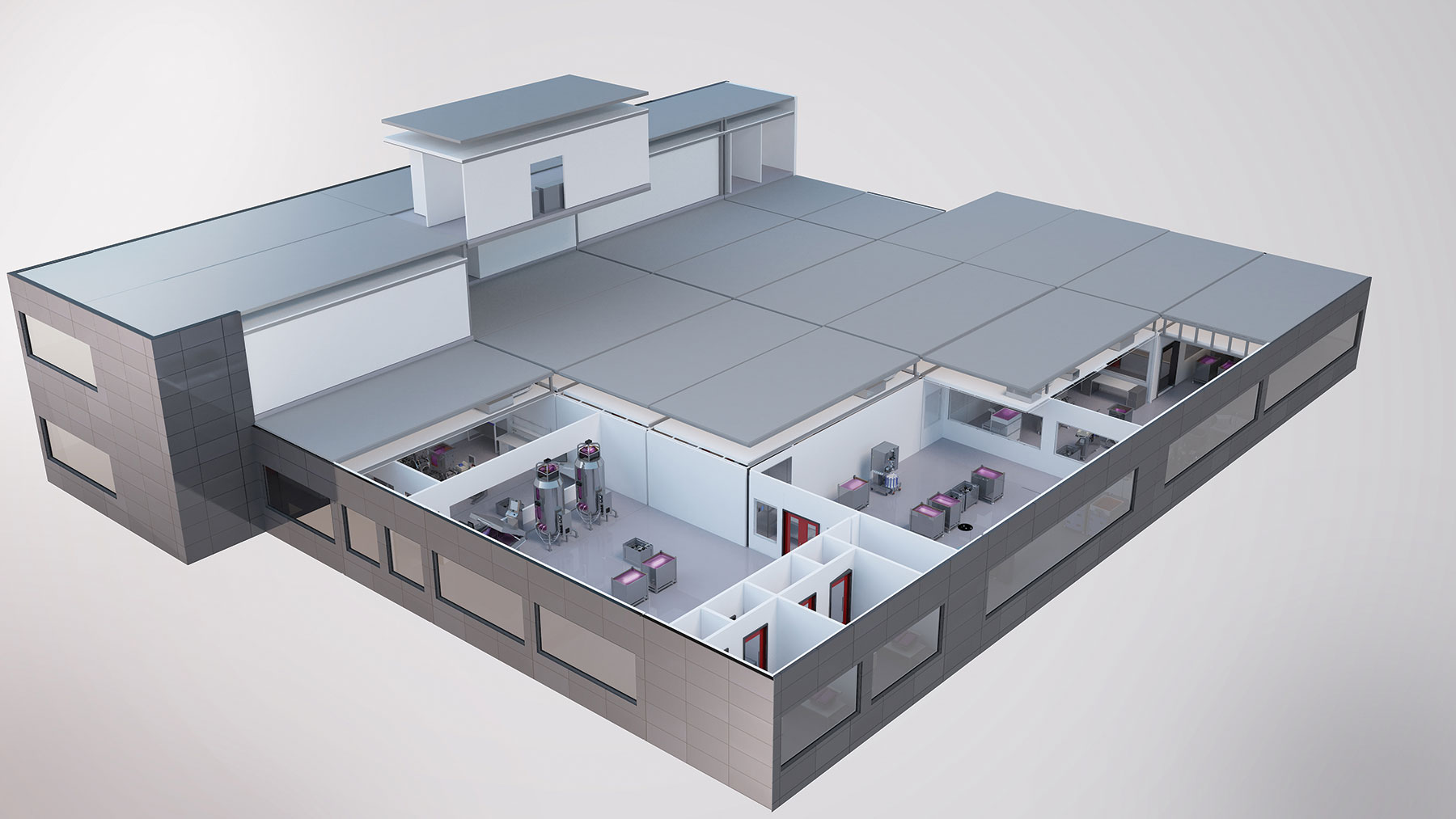
GE Healthcare Life Sciences has spent the past few years finding alternative ways to quickly establish flexible, multiproduct biomanufacturing facilities in emerging markets that traditionally could take years to build.
What Comes Next?
Currently, GE’s KUBio is specifically designed for the manufacture of mammalian cell-based biopharmaceuticals, specifically monoclonal antibodies. The FlexFactory platform can be applied to the manufacture of other types of biologics, for example, vaccines. Many countries are interested in becoming self-sufficient in the production of these important therapies. A KUBio-style solution for vaccines is easy to envision as it would enable enhanced access to much-needed vaccines.
KUBio was indeed a major step forward for flexible, accessible manufacturing solutions, but GE continues to look forward to the future needs for biomanufacturing. Biopharmaceutical companies want to reduce their risks by delaying capital investment until the success of the drug is more clearly defined. Furthermore, they are looking for ways to reduce the capital and operating expenses. GE is addressing this need with a new concept in biomanufacturing that provides flexibility as well as optionality.
In September 2016, GE Healthcare Life Sciences announced GE BioPark Cork, a GE-managed campus including four fully equipped KUBio factories owned by independent biopharma companies manufacturing proprietary medicines. This design and service offering drives further efficiency, beyond that of a KUBio, by centralizing common, non-value-added services like warehousing, utilities, and hydration into a campus structure.
GE’s extensive proven experience in each of these areas brings additional value to customers. Capital investment and speed are both improved by placing a KUBio onto a campus that already has the infrastructure in place to manage the complete manufacturing supply chain for a biopharmaceutical. The $167 million investment in Ireland will create up to 500 jobs and allow biopharma companies to make more nimble decisions and deliver great therapies to patients all over the world.
David Radspinner, Ph.D. ([email protected]), is general manager BioPark, GE Healthcare Life Sciences.







