December 1, 2016 (Vol. 36, No. 21)
Companies Are Now Reporting Some Successes in Preclinical and Clinical Studies
Whether they are proliferating here or invading there, cancer cells can exploit well-evolved moves to evade immune detection.
One way is by deregulating checkpoints, receptor molecules on immune cells that inhibit immune cells from appropriately killing a “foreign” invader.
For example, programmed cell death protein 1 (PD-1) is a T-cell protein receptor normally activated upon binding to PD-L1 ligand (programmed cell death ligand 1) on a non-foreign cell. The PD-1/PD-L1 bond deactivates the T-cell, stopping it from attacking the normal cell.
Some cancer cells also have PD-L1 ligands to co-opt this checkpoint, using it to either escape T-cell attack, or to activate the T cell’s own apoptosis switch causing that T cell’s self-destruction, thus helping to optimize the tumor microenvironment for tumor growth.
Producing focal adhesion kinase (FAK), a nonreceptor (cytoplasmic) tyrosine kinase is another way cancer cells evade immunological attack. FAK acts to phosphorylate proteins and mediates downstream signaling of growth factor receptors and integrins (proteins that help build stroma by attaching cell cytoskeletons to extracellular matrices).
Elevated FAK activity in human cancer cells correlates positively with high fibrosis and low CD8+ cytotoxic T cell (cancer cell killers) penetration in the tumor microenvironment. Cancer stem cells need FAK to survive and initiate tumor growth; it is an important enzyme for tumor migration, invasion, and epithelial to mesenchymal transition which is needed for metastatic growth.
Companies working on immunomodulators to circumvent a cancer cell’s ability to evade immune system attack are reporting success in preclinical and clinical studies when these immunotherapies are administered either alone, or with existing cancer agents and other cancer-fighting modalities.
Verastem is developing FAKi (Focal Adhesion Kinase inhibitors), which reduce immune-system suppressing cells in the tumor microenvironment, such as T regulatory cells (Tregs), myeloid-derived suppressor cells, and M2 tumor associated macrophages, and reduce high-density stroma that can physically block CD8+ cytotoxic T cells from the area.
The company’s FAK/PyK2 Inhibitor program consists of VS-4718 and VS-6063, two immunotherapies that stimulated T-cell growth in human lymphocytes from healthy donors, in contrast with other protein kinase inhibitors, and also induced a dose-dependent reduction of immune cell exhaustion markers, PD-1 and LAG3 (lymphocyte-activation gene 3), on human CD8+ T cells.
In a preclinical model comparing immune-deficient nude mice with immune-competent mice, FAK knockout-induced tumor regression dependent upon a functioning immune system, CD8+ cytotoxic T-cells for infiltrating and killing tumors, and reduced Tregs.
A pancreatic cancer transgenic mouse study showed that FAKi combined with gemicitibine, the anticancer drug, extended animal survival compared with controls. The greatest increase in survival occurred when FAKi was administered with gemicitibine and anti-PD-1. FAKi also reduced stromal density, which correlated positively with increased infiltration by CD8+ T cells.
Clinical trials are underway to test efficacy of FAKi in combination with anti-PD-1 and anti-PD-L1 monoclonal antibodies.
“The holy grail for Verastem,” said Jonathan Pachter, Ph.D., CSO, “is to increase the duration of response for patients receiving other anticancer therapies, including immune checkpoint inhibitors by increasing CD8+ T-cell infiltration into tumors, and decreasing immunosuppressive cells in the tumor microenvironment.”
Progenra develops medicines based on the ubiquitin proteasome pathway. This highly regulated pathway is required to catabolize short-lived proteins that modulate cell division and proteins responsible for correct protein folding. Pathway defects cause pathologies.
Ubiquitin-specific-processing protease 7, (USP7), is a key modulator in several cancer and viral signaling pathways such as DNA damage response, immune response, and tumor suppression. Tregs need USP7 for proper functioning. Its overexpression correlates significantly with a malignant phenotype, and it is a predictive factor for poor prognoses in hepatocellular carcinoma and non-small cell lung cancer.
USP7 as a prognosticator and potential therapeutic target for these two diseases spurred Progenra scientists to create USP7i (inhibitors) to impair Tregs’ functions and promote effector T-cell functions.
Progenra’s USP7i target tumors and the immune system as a dual activity concept: P5091 is a selective USP7i that inhibits growth of multiple myeloma, MM1.S, and P22077 inhibits neuroblastoma growth.
“USP7i are unique in that they can exert direct antitumor activity and enable the patient’s immune cells to kill the tumor,” explained Suresh Kumar, Ph.D., director of R&D.
These two USP7i block formation of the USP7-ubiquitin-vinyl-methyl-ester complex, which suggests that they target USP7’s active site. A two-hour exposure of USP7i in Jurkat cells, an immortalized line of human T-lymphocytes, led to sustained inhibition of USP7 activity. Mouse Tregs pre-treated with 10 µL USP7i for two hours, then washed and co-cultured with T effector cells showed that Treg function impairment.
Risks and Rewards
In terms of the USP7i’s potential risks and rewards, in molecular-based therapy, whether it entails classic receptor agonism/antagonism, or inhibition or activation of key enzymes, there is always the possibility that the therapeutic molecule will act on targets other than the ones intended, leading to unwanted side effects, according to Dr. Kumar.
“Most small molecules have the chemical potential to interact with multiple targets. The key is to find molecules that act on only the intended target at low doses. To the extent that this isn’t possible, it is necessary to manage any side effects clinically,” he says.
Dr. Kumar sees some of Progenra’s compounds best suited to combination therapies such as in immuno-oncology (IO) protocols, in which cancer can be treated with a reduced likelihood of the tumor developing resistance to the therapy. There is also a potential benefit with developing a small molecule.
“Treating patients with small molecules rather than expensive biologicals is highly cost-effective, providing therapy to all patients rather than a small group,” noted Dr. Kumar. “Pharmacies and patients are better adapted to pills and capsules than to expensive injectable biologicals.”
In considering clinical trials for these dual-activity IO therapies, Dr. Kumar thinks that tumors with higher USP7 expression levels are likely to be more susceptible to direct antitumor effects compared with those with low levels. On the other hand, highly immunogenic tumors that have a high Treg presence may benefit from the immunotherapy-based mechanism.
He sees a challenge in selecting appropriate combination agents for clinical work. “In preclinical studies, Progenra USP7i worked well in combination with IO, such as anti-PD1 antibodies and vaccines. Developing appropriate conditions for combination therapies to achieve the best therapeutic index possible will be challenging because of the increased complexity,” he says.
Checkmate Pharmaceuticals is increasing checkpoint inhibitor efficacy with a toll-like receptor (TLR) agonist delivered in a virus-like particle (VLP) that turns “cold” tumors into “hot” tumors, to increase immune cell infiltration and engagement.
The company has an exclusive oncology license from Cytos for CMP-001, a VLP made up of a viral protein and CpG-A. The latter is a bacterial DNA sequence containing unmethylated CpG and is a potent, innate immune-system inducer.
“The VLP induces production of antibodies against itself,” stated Arthur Krieg, M.D., Ph.D., Checkmate’s CEO, “but in contrast to neutralizing antibodies (Abs), these Abs serve two key roles in tumor rejection. First, the Abs form immune complexes in the tumor that provide an added immune stimulatory function. Second, immune complexes are taken up by the plasmacytoid dendritic cells (pDCs) through their activating FcgRIIa (Fc receptor, CD32), which induces an unprecedented level of pDC immune stimulation.”
CpG-A’s DNA sequence differs from all other immune activators, noted Dr. Krieg.
“CpG-As polyG motif forms G quadraplexes—large multimeric complexes that engage toll-like receptor (TLR)9 in a unique way—leading to unparalleled levels of type 1 interferon (IFN 1) production from pDC,” he continued.
TLR9 recognizes unmethylated CpG sequences in DNA, and TLR9’s signals activate cells that initiate pro-inflammatory reactions. Immature pDC are in many tumors and maintain immune tolerance by supporting Treg and myeloid derived suppressor cells.
“We believe that activation of these tumor-infiltrating pDC through TLR9 by CpG-A uniquely switches them from an immune-tolerizing state, to an immune stimulating state, in which pDCs pump out massive amounts of IFN and other chemokines and cytokines, recruiting DCs and T cells, and driving a systemic antitumor T-cell response,” said Dr. Krieg.

Checkmate Pharmaceutical’s lead product candidate, CMP-001, is a CpG-A that converts immunologically “cold” tumors to immunologically “hot” tumors.
Controlling Growth of Treated and Untreated Tumors
Checkmate scientists also found that anti-PD-1 intratumoral CMP-001 controlled growth of treated and untreated tumors. A Phase Ib trial of intratumoral CMP-001 with systemic pembrolizumab, a humanized antibody, is underway in subjects with advanced melanoma, who either progressed on pembrolizumab, or who failed to respond to it after more than 12 weeks.
“The first evidence of success in the clinic was the induction of serum T cell ‘helper’ 1 chemokines, IP-10 and MIG, which indicates a strong IFN effect. The second measure of success is clinical response with tumor regression, which we are now seeing in the clinic,” explained Dr. Krieg, who adds that safety is always a potential challenge.
“Many IO agents can induce autoimmune disease and other serious toxicities. Autoimmunity has not been seen in previous CpG-A clinical trials in more than 700 humans, nor in other CpG oligo trials. On theoretical grounds, it’s possible that CpG may aggravate the known autoimmune disease risk from anti-PD-1 checkpoint inhibitors; we will be watching closely for evidence of this.”
Bellicum Pharmaceuticals has developed a proprietary platform based on either an “on” switch, or an “off” switch to control T-cell therapies in vivo. Called chemical induction of dimerization (CID), the technology comprises molecular switches, and modified signaling proteins that are triggered inside a patient by infusing rimiducid, a small molecule that binds to only a specifically designed domain of CID switch proteins.
In the patient, rimiducid dimerizes CID switch proteins creating a cluster that starts the signaling cascade leading to either immune cell activation, i.e., proliferation and survival, or apoptosis, depending on the switch used. These CID-based product candidates depend on cell-signaling molecules.
The “on” switch is inducible MyD88/CD40 (MC), a protein for survival and proliferation. MyD88 (myeloid differentiation primary response gene) protein functions in cellular responses to stress, cytokines, bacteria, and viruses. CD40 is a co-stimulatory protein found on DCs and B cells, and is required for their activation. The “off” switch, caspase-9, is a cell-signaling molecule; when activated, it initiates the apoptosis pathway.
“The platform allows the physician to tailor the immune response to meet the patient’s needs for specific cellular immunotherapy approaches and therapeutic indications,” said Aaron Foster, vp of product discovery For example, CaspaCIDe is a safety switch in the company’s hematopoietic stem cell transplant (HSCT) and T-cell receptor products that is activated if the patient has a serious side effect.
The CaspaCIDe switch is made up of the CID-binding domain coupled to the signaling domain of the caspase-9 enzyme. Rimiducid infusion triggers activation of the caspase-9 (iCasp9) domain which, in turn, leads to selective apoptosis of the CaspaCIDe-containing cells.
“CAR T cells have led to some toxicities, including cytokine release syndrome,” explained Foster, “so the activation state of the T cell needs to be managed. There must be a fine balance that can be monitored and controlled.”
GoCAR-T is based on the iMC “on switch” and comprises modified CAR T cells and MC. In this product, however, MC is a molecular switch whose expression drives CAR T-cell activation and expansion through a scheduled course of rimiducid infusions. If side effects occur, GoCAR T-cell activation is lowered by reducing the rimiducid administration schedule.
“In this way,” Tom Farrell, Bellicum’s president and CEO, explains, “we’re placing co-stimulation, which is responsible for proliferation and survival of the T cells, under the physician’s control, rather than leaving it dependent upon antigen, which is unpredictable and uncontrollable. That’s ‘co-stimulation on demand.’”
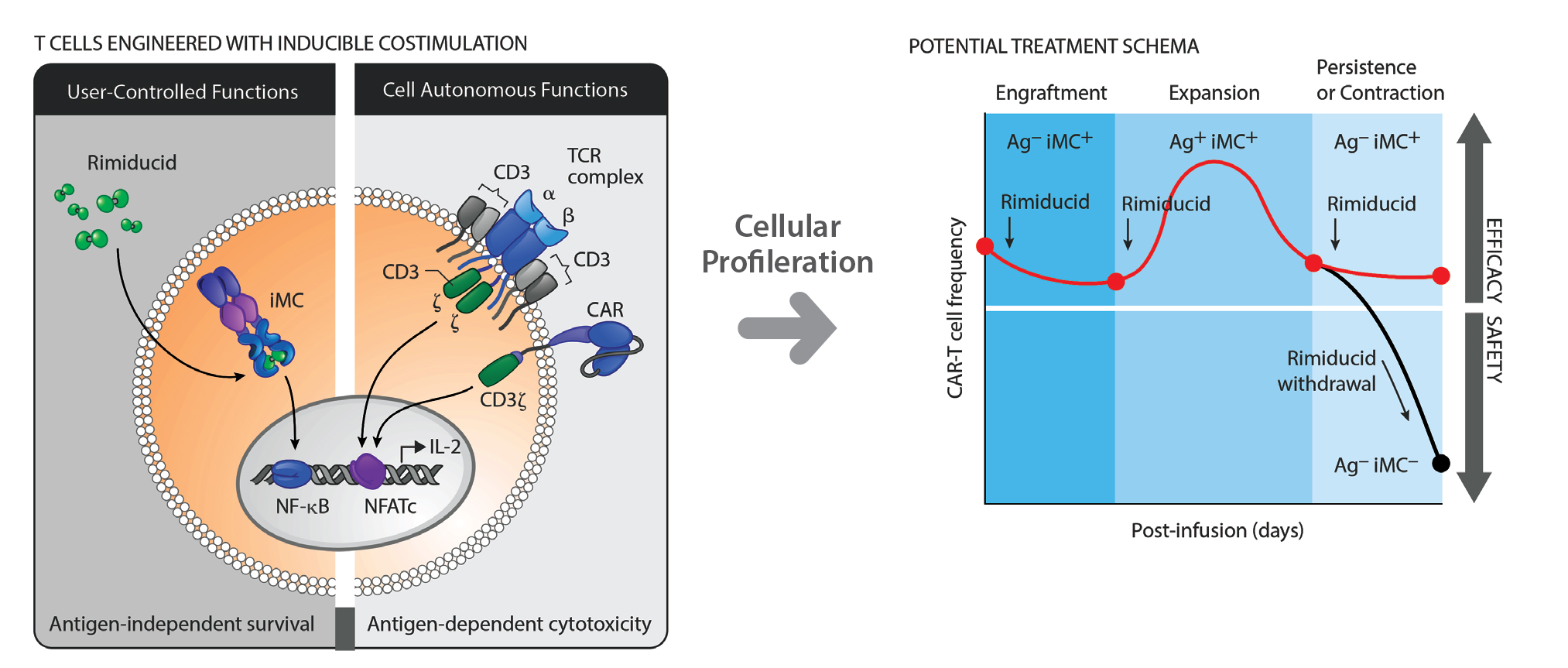
Bellicum Pharmaceutical’s GoCAR-T technology incorporates a switch that activates CAR T cells when triggered by both rimiducid and the targeted antigen expressed on the surface of the cancer cells. [Illustration by www.justinkleinCMI.com]
Facilitating the Development of Complex Proteins for Immuno-oncology
In the rapidly emerging field of immuno-oncology, the focus is on four major areas of active protein pharmaceutical drug development: vaccines, cell therapies (CAR T-cell and T-cell receptor engineering), bispecifics, and checkpoint inhibitor antibodies. These protein classes and novel protein fusion molecules are identified by how they activate and steer the immune system toward tumor-specific targets. This new path towards controlling cell growth through the immune system offers great hope for the clinic.
Once the novel immuno-oncology drug is identified in the laboratory and its biological properties validated, the drug development funnel tends to be extra difficult for these classes of molecules. Can the protein be expressed in sufficient quantities to support clinical development? Does it aggregate? Is it stable at high concentrations? For how long does it remain active?
When the functional properties of the antibody are not easily selected from large libraries of variants, such as affinity or specificity for an antibody, what is one to do? When you can only screen 24–96 variants for the developability properties, how do you choose what variants to look at? The potential sequence space is vast. Exploring every single amino acid substitution independently is costly, and exploring all possible pairs and triplets is almost impossible.
Officials at DNA2.0 say they have developed tools and technologies such as gene synthesis, codon optimization algorithms, expression vectors, and rapid expression systems to produce thousands of proteins at a time and within weeks. They note that the integrated platform allows them to deploy design of experiment (DOE) approaches in combination with machine learning algorithms to design and make protein variants in a systematic and reiterative approach and rapidly characterize these sets of variants for developability properties.
For example, a novel immunotherapy approach combined an antibody and a cytokine into a single moiety. The fusion protein expressed poorly, aggregated, and had diminished biological activity. Using DOE to design a set of variants allowed company scientists to investigate multiple properties in each variant and regardless if the variant is positive, negative, or neutral in its effect on the properties, provide information for machine learning to design a second set of variants focused on exploiting positions and compositions in the protein that had a favorable developability profile identified in the more laborious characterization assays.
Machine learning helps scientists rescue exciting lead molecules in the immuno-oncology pipeline and engineer them for industrial development.



