January 1, 2010 (Vol. 30, No. 1)
Amy MacLauchlan
Ernie Jenness
Kerry Roche Lentine
Vikas Gupta
Important Considerations Include Robustness of the Design and Sterility Assurance Levels
In the last few years, the biotech industry has witnessed the rapid adoption of single-use technology in bioprocess manufacturing. Concurrently, there has been a strong push to reduce operating costs by moving manufacturing from traditional stainless steel equipment in a cleanroom to single-use disposable solutions in a “gray space” environment. As contamination risk increases exponentially in gray space environments, it has become imperative to ensure a validated sterile flow path for single-use solutions.
Presterilized single-use connectors perform the critical function of connecting two presterilized single-use components in a nonsterile environment, while maintaining fluid-path sterility. As a result of the increasing use of single-use connectors, many new types of connectors have entered the marketplace. At the same time, their use has expanded beyond the typical upstream buffer and media applications, into high value-added downstream applications like ultrafiltration/dialfiltration and final fill and finish. This makes it extremely important that the choice of a single-use connector should take into consideration robustness of the connector design and sterility claims made by the vendor.
By design, a sterile connector should protect the fluid path by never exposing it to the open environment. For example, Millipore’s Lynx® S2S connector employs a solid plug, not a membrane or peel strip, that could create a gap.
When making sure that a single-use connector maintains a sterile fluid path, it is important to verify that the methods used to support these claims are valid, reproducible, and provide meaningful results. It is equally critical to ascertain that the selected connector will secure the fluid path at a sterility assurance level (SAL) of 10-6 under the most rigorous conditions.
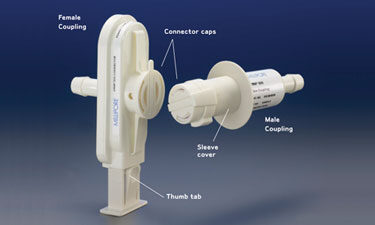
The Lynx S2S connector is constructed of a female coupling and a male coupling.
Lynx S2S Connectors
The Lynx S2S connector is a single-use, single actuation device. It is composed of a male and female coupling set (Figure) designed to maintain sterility during the connection of two independent sterile fluid paths. Both units of the connector use a hose barb fitting to connect desired bags, containers, or assemblies to the fluid path.
Each fluid path is then sterilized by either gamma radiation or by autoclave in preparation for a sterile transfer of media, buffer, or even final drug product. The connector’s process contact materials are constructed of high-temperature gamma-stable polysulfone with an over-molded silicone gasket, allowing for autoclaving up to 130°C for 30 minutes and/or gamma irradiation up to 45 kGy.
The male and female coupling sets of the Lynx S2S connector uses solid plugs with silicone o-ring seals and gaskets for containment. This design provides high-pressure sealing and a tortuous path that prevents passage of bacteria. After the couplings have been autoclaved and/or gamma irradiated, two sterilized assemblies can be connected by a series of simple steps in any environment.
During the connection process, the plugs, which will become contaminated in a nonclassifed area, are isolated by moving them up and away from the fluid path within the female coupling body. This process assures a sterile fluid path (SAL of 10-6) for the liquid to flow through the connection.
Aerosol Challenge Studies
Millipore uses an aerosolized bacterial challenge test to simulate worst-case environmental contamination. This test challenges the Lynx S2S connectors with greater than one million bacteria (in colony-forming units) per device, thus providing the most stringent testing in the industry.
In this test, 30 connector coupling sets, 10 each of the Lynx S2S connector and 10 each of two competitors, were challenged with an aerosolized suspension of Brevundimonas diminuta at equal to or greater than 106 colony-forming units.
The connector coupling sets were connected and actuated in this aerosolized environment. Sterile broth was transferred through the connectors into sterile receiving vessels, then assayed to determine the presence or absence of growth. Device controls were employed to assure proper execution and function of the test.
The negative device control consisted of a connector coupling set, which was connected and actuated within the isolation chamber without being exposed to the challenge organism; the positive device control consisted of a connector coupling set with its barrier film, membrane, or plugs removed prior to being exposed to the challenge organism, and then connected and actuated. The collection vessels and negative and positive controls were incubated and scored for presence or absence of growth for over a five-day period. Samples exhibiting growth were confirmed to be the test microorganism B. diminuta.
Results
All 10 of the Lynx S2S connectors passed this test. Only 2 out of 10 competitor A connectors and 2 out of 10 competitor B connectors passed the test, representing an 80% failure rate. Control results were as expected.
The design of the Lynx S2S connector has demonstrated a quality and robustness that will provide significant benefits to the industry by facilitating sterile transfer of fluids with a high degree of security and safety. One of Millipore’s Mobius® flexible bioprocessing solutions, the Lynx S2S connector delivers safer and robust connection for all unit operations within the biopharmaceutical manufacturing process.
Amy MacLauchlan ([email protected]) is validation engineer, microbiological sciences, Ernie Jenness is product manager, Kerry Roche Lentine is manager, virology and microbiological sciences, and Vikas Gupta is product manager at Millipore. Web: www.millipore.com.