November 1, 2007 (Vol. 27, No. 19)
Mark A. Collins
New Tools Designed to Improve the Efficiency of High-content Screening Are Available
As the industry moves to further streamline R&D and tackle the ever growing attrition rates of compounds, there is much focus on gathering richer data about targets, disease pathologies, and ADME/Tox profiles much earlier in the drug discovery process.
High-content screening (HCS), which directly addresses these key drivers, has emerged as a central approach for discovery research. The ability of HCS to measure multiparameters related to spatial, temporal, and intensity changes in populations of individual cells allows scientists to visualize and quantify the real effects of an experiment from a system perspective rather than from a single data point of a homogeneous system.
Technology Evolution
Given its real value to the discovery scientist, the demand for more advanced HCS systems continues to increase.
Thermo Fisher Scientific (www.thermo.com) has responded with a fully integrated HCS solution that includes imaging technologies, fluorescent reagents, engineered cells, image analysis software, data-management, automation, informatics, and bioinformatics tools.
In this article, we look at how the combination of advanced imaging technology and intelligent software can be implemented to alleviate the assay development bottleneck and improve data quality in HCS screens.
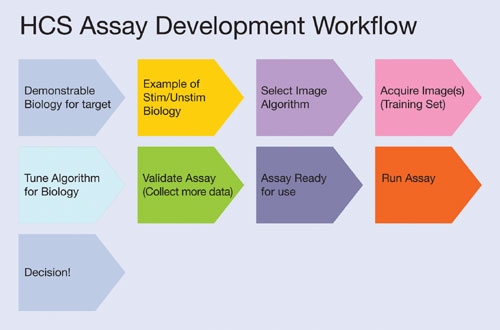
Figure 1
Alleviating Logjams
Once the biology of the assay has been developed and the effects visualized by eye, the scientist must translate this into a robust, quantitative assay with a decent assay window. This is commonly problematic as the researcher must first have examples of positive and negative biologies, decide the cells and target(s) of interest, acquire the image(s), choose a suitable set of measurements, make those measurements, analyze the data from this image, and then return to the microscope to tune the set-up around the process again (Figure 1).
Usually this occurs with only one small set of images. Ensuring assay robustness over numerous plates and hundreds or thousands of wells requires further optimization that is best achieved by a more data-driven rather than an image-driven process. Thus, an HCS system that allows a more rapid assay development process will overcome the assay development bottleneck more efficiently and have an overall larger impact on productivity in HCS.
Furthermore, the efficient management of cell cultures and assay treatment will not always mitigate assay variability, a phenomenon that is often introduced through pipetting error or patchy cell growth. These effects can have a significant impact on the response of cells in different areas of any one well. The provision of an intelligent system that can detect and compensate for this variability would improve data quality and overall workflow efficiency.
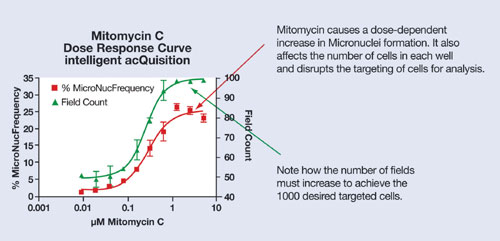
Figure 2
Intelligent Software
It is the ability to quantify the biology of an assay, and therefore provide meaningful biological results, that dictates the usefulness and success of an HCS assay. Performing those steps in as efficient a manner as possible is what we define as rapid assay development (RAD).
The Thermo Scientific modular Cellomics® HCS platform with ArrayScan® HCS reader can eliminate assay development and optimization bottlenecks via the intelligent acQuisition™ (iQ) approach, which couples adaptive, intelligent image-acquisition software (Scan) with real-time image analysis from the BioApplication Suite and provides an iterative data-driven approach to assay development and optimization that delivers a RAD approach to HCS.
Using the Thermo Scientific iQ approach, Scan software allows the data-driven tuning of the assay through repetitive cycles of image acquisition, real-time image analysis, data review, and imaging parameter optimization to achieve the desired assay window. Once an assay window is achieved on one or two images, the system is then set up to acquire and analyze a larger training set to check the robustness and biological relevance of the assay.
Visualization of data and image re-analysis can be scaled beyond the instrument through software running on a desktop PC if required. In this way, researchers can develop and further tune assays off-line from the instrument. The system allows the scientist to visualize and identify cell variability and compensate for this within data analysis, i.e., with the exclusion of those cells from selected data sets.
It also allows the user to distinguish between subpopulations of varying activity. Once the criteria for a robust assay are established, the system can be set up to ensure the acquisition and selection of the correct number of targeted cells for the biology in question, irrelevant of plating density and compound effects, and then deliver the images and data for decision making.
As many scientists incorporate numerous assay types into their screening workflow, it is advantageous to have an assay platform that is flexible enough to accommodate them all. Thus, a system that allows the measurement and quantification of a plethora of cellular events such as changes in cell morphology, cell viability, mitosis, apoptosis, and cell-signaling pathways is beneficial.
The Thermo Scientific Cellomics BioApplications software, which is integrated into HCS platform, is a series of image-analysis software algorithms developed and validated for the quantitative measurements of almost any biology—the relevant program can be selected and run depending on the assay of interest.

Figure 3
Improved Toxicity Models
To counteract the number of new chemical entities that have been failing in late-stage development, scientists have been looking for new and robust toxicity assays, and HCS is becoming a tool of choice for toxicity profiling. With each of these toxicity assays comes differing challenges.
For rare event assays that are used to measure genotoxicity, it is essential that enough targeted cells are measured to meet stringent regulatory guidelines. To aid scientists with accelerating the genotox profiling of compounds, Thermo Fisher Scientific applies its iQ approach and software. RAD allows assays to be optimized.
Once a Genotox assay is developed, scientists can define criteria to collect just the right amount of targeted cells to ensure high quality yet statistically relevant data. As the dose of the compound increases, iQ allows the Cellomics HCS system to adaptively respond and collect the right number of targeted cells regardless of dose (Figure 2). Systems that collect a fixed number of images and have no adaptive capability will either under-collect cells leading to unreliable data and a need to perform the assay again or over-collect data leading to additional data storage and increased assay time (Figure 3).
Additionally, the iQ approach is suited to assays where the number of cells is not proscribed ab initio but where obtaining the right number of cells is still key, e.g., a cell-cycle analysis assay. Here the scientist is able to maintain a robust assay window using the iQ approach, even when screening compounds over dose ranges that may cause increasing levels of cell death (Figure 4). iQ ensures that the right number of targeted cells can be obtained and that assay Z´ scores are optimized.
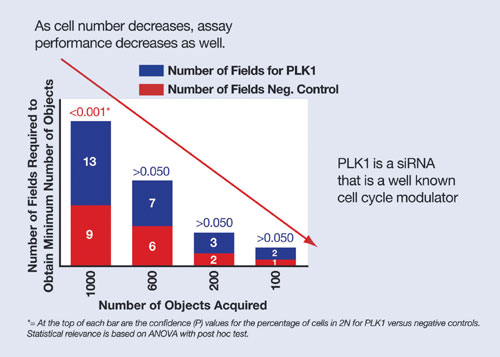
Figure 4
Mark Collins, Ph.D., is senior manager, marketing, laboratory automation & cellular imaging, at Thermo Fisher Scientific. E-mail: [email protected].







