September 1, 2012 (Vol. 32, No. 15)
Protein profiling has existed in one form or another for several decades and has its origins in enzyme purification strategies of the mid 20th century.
The advent of computational technologies in biomedical science has catalyzed the development of myriad high-throughput experimental platforms and the birth of the “omics” age.
The proteome represents the identity, expression levels, interacting partners, and post-translational modifications of proteins expressed within any given cell. Global protein profiling—or proteomics—aims to census the quantitative and qualitative factors regulating the biological relationships of proteins acting in concert as functional cellular networks.
In addition to the broader, discovery-driven scope of proteomics, however, more focused protein-profiling approaches are still widely used to validate hypotheses and add finer brush strokes to the mechanistic and functional characterization of protein biology. This year’s “American Association of Cancer Research” conference included sessions focusing on the application of protein-profiling techniques and strategies for basic and translational aspects of cancer biology.
“Chemotherapeutic sensitivity encompasses both sensitivity of the tumor resulting in response to therapy and sensitivity of the individual to the toxic side effects of the chemotherapy,” said Eileen Dolan, Ph.D., professor of medicine at the University of Chicago.
“Identifying individuals with genetic variants that make them more sensitive to a particular adverse event associated with chemotherapy, or less likely to respond, will help clinicians provide alternative therapies or a lower dose of chemotherapy to prevent toxicity.”
Dr. Dolan’s group is interested in characterizing the role of transcription factors and signaling molecules in mediating chemotherapeutic sensitivity, with a view of ultimately harnessing these proteins as novel therapeutic leverage points. Dr. Dolan’s colleague, Richard Jones, Ph.D., an assistant professor in the University of Chicago’s Ben May department for cancer research, described a novel technique, referred to as microwestern, that the group is using to identify candidate proteins.
“Microwestern technology can be thought of as DNA expression arrays for proteins with a twist,” said Dr. Jones. “The twist is that biological samples examined with the platform undergo a chromatographic separation following their deposition onto microwestern gels, whereby small proteins migrate through a gel matrix at a quicker speed than do large proteins.”
While DNA microarrays work well through the exquisite selectivity afforded by the predictable complementary base pairing between labeled mRNAs and immobilized DNA oligonucleotides, there is no comparable complementarity for proteins, and unique affinity reagents must be designed specifically for each protein that a researcher wishes to pursue.
Dr. Jones cited two disadvantages of existing Western-based protein profiling that microwesterns address. “Antibodies obtained through traditional processes typically cross-react to some degree with proteins other than their intended targets. In addition to this, standard Western blots require the use of large amounts of cellular material, large amounts of antibody reagents, and a large amount of human intervention.
“By electrophoretically resolving microarrayed cell lysate proteins through a gel matrix, protein isoforms representing intended targets can be visually discerned from unintended targets. Microwesterns also reduce starting material and antibodies required by up to two orders of magnitude.” Dr. Jones added that microwesterns can outperform mass spectrometry-based methods in metrics of sensitivity and reproducibility of detection of low-abundance proteins.
“The ability of the microwestern array to measure the levels of large numbers of unique proteins across populations of individuals makes it a wonderful platform for the identification of variants that affect protein function,” noted Drs. Jones and Dolan.
The group applied the technology in a study that evaluated the relationship between protein abundances, genetic variation, microRNA and mRNA expression, and chemotherapeutic-induced cytotoxicity and apoptosis in lymphoblastoid cell lines. They identified over 70 proteins whose levels were associated with cytarabine-induced cytotoxicity and apoptosis, knockdown of one of which, ATF2, was associated with a restoration of apoptosis in response to the drug in the cell lines.

A micro-western array chip allows for 96 miniature Western blots to be performed quickly and efficiently within the context of a 96-well microtiter plate. 50 nanoliters of cellular material are microarrayed along with a protein molecular weight standard into each well of the plate and then 250 nanoliters of primary and secondary antibodies are used to detect changes in the relative abundance and modification state of proteins. With the microwestern array method, a single technician can reportedly perform analysis of over 1,000 unique proteins across many conditions within the course of two weeks. [University of Chicago]
VEGF and Drug Resistance
VEGFA (or simply VEGF) is a master regulator of angiogenesis, a critical process in tumor development, and is well established as a mitogen, survival factor, and permeability factor for endothelial cells. A group led by Gillian Tozer, Ph.D., of the University of Sheffield is using mouse models of cancer to shed light on the role that VEGF isoforms play in mediating resistance to therapeutic regimens.
“The most common splice variants in mice are VEGF120, 164, and 188, and these different isoforms have unique roles in tumor vascular maturation processes. For example, VEGF188 has been found to be associated with a relatively mature tumor vasculature compared with the other main isoforms in the mouse,” Dr. Tozer explained.
Her group recently found that tumor expression of VEGF120 in mice makes tumor vasculature particularly susceptible to damage by the tumor vascular-disrupting agent, combretastatin A4 phosphate (CA4P) but, compared to VEGF188-expressing tumors, resistant to radiotherapy.
To identify potential players mediating these discrepancies, the group carried out differential proteomic profiling of wild-type and VEGF isoform-specific tumor cell lines and solid tumor lysates using commercial angiogenesis and phosphoreceptor tyrosine kinase arrays (R&D Systems) combined with iTRAQ technology (Life Technologies).
They found that fibroblast growth factor (FGF-2), neuropilin 1 (NRP1), and platelet-derived growth factor receptor (PDGFR) were upregulated in VEGF188-specific tumor cells, while Neuropilin 2 (NRP2) was downregulated in the same tumor extracts relative to VEGF164 and VEGF120. While stressing that VEGF-based therapeutic strategies are in the early days of development, Dr. Tozer does see promise ahead.
“In Roche and Genentech’s AVAGAST (gastric cancer) trial, the ELISA was more sensitive to low molecular weight VEGF than 165 and 189, and when used to screen blood samples, was capable of predicting which patients would benefit from bevacizumab treatment, i.e., the higher the levels, the more benefit,” she pointed out.
Studying EMT in Breast Cancer
In addition to global proteomic screening strategies, protein profiling also encompasses more focused and informed experimental strategies. Epithelial to mesenchymal transition (EMT) refers to the process by which epithelial cells lose their cell-cell junctions, exhibit spindle cell morphology, and acquire increased cellular motility.
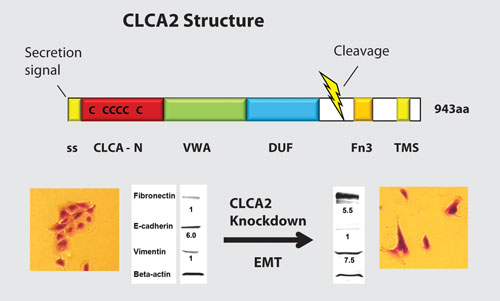
In breast cancer, EMT facilitates invasion of surrounding tissues and correlates closely with cancer invasiveness, metastasis, and relapse. A group led by Randolph Elble, Ph.D., of Southern Illinois University’s School of Medicine is focusing on the role played in EMT by members of the hCLCA family of calcium-activated chloride channel regulators.
In addition to the role suggested by their name, these proteins also function as secreted metalloproteinases, and their loss indicates an increased risk of metastasis. “Our studies were based on observations of apparent EMT in knockdown cell lines,” he explained.
“To determine whether attenuation of CLCA2 and 4 really caused EMT, we assessed expression levels of proteins associated with epithelial or mesenchymal phenotypes by Western blot.”
The transition from epithelial to mesenchymal phenotype takes about two weeks following knockdown of CLCA2 or 4, and can be confirmed by observing delocalization of E-cadherin from cell-cell junctions. “We could quantify proteins over a wide range of expression using a Li-Cor Odyssey fluorescent scanner,” said Dr. Elble.
The group found that loss of these proteins is associated with transition to a mesenchymal phenotype in breast cancer, raising an inherent paradox: “Most metalloproteases promote the very processes that CLCA2 and 4 inhibit,” Dr. Elble pointed out.
He anticipates that the work may have considerable clinical payoff. “Clinically, we found that the loss of CLCA2 expression in primary breast tumors presages a greater risk of metastasis, and we are currently investigating the therapeutic potential of the secreted form of the protease.”
Researchers at Southern Illinois University’s School of Medicine are studying the role played in epithelial to mesenchymal transition (EMT) by members of the hCLCA family of calcium-activated chloride channel regulators.
Manipulating Protein Concentration
In the absence of a sufficiently specific and sensitive clinically approved biomarker for cancer, current methods for diagnosing neoplastic diseases are largely restricted to costly imaging-based procedures, such as computer-assisted tomography or magnetic resonance imaging.
Mauro Ferrari, Ph.D., is president and CEO of the Methodist Hospital Research Institute (MHRI) and professor of internal medicine at Weill Cornell Medical College. Dr. Ferrari and his colleague, Tony Hu, are developing a novel technology that enriches low molecular weight proteins for biomarker discovery.
“Low molecular weight and abundance proteins are excellent candidates for biomarker development, but these proteins can be a billion-fold less concentrated than the most abundant proteins,” said Dr. Ferrari. “In other words, highly abundant proteins often interfere in the detection of lower abundant proteins.”
By precisely controlling the pore geometry and surface chemistry, the MHRI/Weill label-free nanopore-based assay can selectively sort and concentrate proteins of interest from blood samples. Tumor samples are spotted onto a nanoporous silica layer, in which small peptides are trapped, while the larger proteins are removed from the surface after washings. Captured molecules can then be eluted from the pores and analyzed by MALDI TOF MS.
“With no need for additional sample-preparation steps or invasive procedures, this platform provides a rapid, reliable, and low-cost method for novel biomarker discovery,” Hu said.
The nanopore assay has been used to identify subtle changes in low molecular weight protein expression signatures using negative control and diseased serum samples collected from imaging-proven stage I–III cancer mice models, and the application of mass-spectrometry allows for the identification of proteins unique to different stages of cancer development.

Schematic workflow for nanopore-based proteomic profiling. [Ye Hu, Methodist Hospital Research Institute]



