July 1, 2013 (Vol. 33, No. 13)
Optimizing the Process in an E. coli System with Xbrane Lemo and Rhamex Technologies
Escherichia coli is one of the most widely used hosts for the production of proteins. However, the optimization process usually presents a challenge.
To address this issue, Xbrane Biosciences developed the Lemo and Rhamex systems. Both systems allow optimizing the production of both routine and difficult targets in E. coli. They also play a central role in OptiXpress protein production screening and strain development services.
In many E. coli protein production strains, the expression of the gene encoding the target protein is driven by the T7 RNA polymerase (RNAP). Finding the expression level of the gene encoding the target protein that results in optimal protein production yields is usually based upon time-consuming and laborious screens involving many different strains and culture/induction regimes.
The Lemo21(DE3) strain is derived from the widely used T7 RNAP-based strain BL21(DE3), which harbors the pLemo plasmid (Figure 1). The gene encoding the T7 RNAP is located on the chromosome. Its expression is governed by the isopropyl b-D-1-thiogalactopyranoside (IPTG) inducible lacUV5 promoter, which is only poorly titratable.
The activity of T7 RNAP can be modulated by expression of the gene encoding the natural inhibitor of the T7 RNAP— T7 lysozyme—from the pLemo plasmid. The pLemo plasmid has a p15A ori, making it compatible with any pET vector, and a chloramphenicol resistance marker.
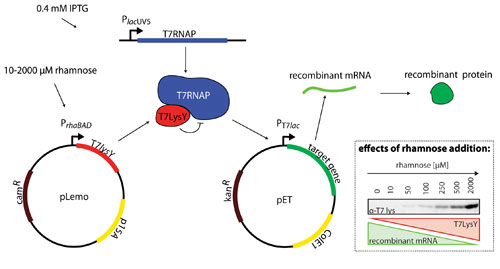
Figure 1. Regulating target gene expression levels using the Lemo21(DE3) strain
Well-Titratable System
Expression of the gene encoding the T7 lysozyme is governed by the well-titratable rhamnose promoter system. The gene encoding the target protein is located on a pET-vector. In the example shown here, the pET-vector has a kanamycin resistance marker. The expression of the gene encoding the target protein from the pET-vector is governed by the T7lac promoter.
In the Lemo system the expression levels of the gene encoding the target protein can be regulated by adding different amounts of rhamnose to the culture. The more rhamnose added, the more T7 lysozyme is synthesized (Figure 1, right).
As a consequence, T7 RNAP activity is increasingly inhibited, and the expression levels of the target gene decrease (Figure 1 inset), thus allowing the identification of the gene expression level for the optimal production of a given target. Lemo21(DE3) is fully compatible with all widely used culture media, including autoinduction based media, and culture set-ups, including fed-batch.
As shown in Figure 2, Lemo21(DE3) makes it possible to identify gene expression levels for the optimal production of any protein using one strain and a limited number of culture/induction conditions. Importantly, at the optimal rhamnose concentration the population of cells in the culture remains completely homogenous; i.e., all cells produce the target protein.
In Figure 2A the production of the polytopic membrane protein YidC was optimized in Lemo21(DE3). To monitor production levels of functional membrane protein material, green fluorescent protein (GFP) was fused to the C-terminus of YidC. Protein GFP-fusions allow the use of whole cell fluorescence to rapidly and accurately monitor protein production levels.
Cells were cultured in Lysogeny Broth (LB) in the presence of different concentrations of rhamnose, and the expression of the gene encoding the T7 RNA polymerase was induced with 0.4 mM IPTG. In Figure 2B the production of a set of proteins in BL21(DE3) and Lemo21(DE3) was compared.
The target proteins are the bacterial polytopic membrane proteins EnvZ, PheP, and YidC; the human polytopic membrane proteins Tetraspanin (Tsp) A and B; the soluble proteins Glutathione S-transferase (GST) and GFP alone. All proteins, except GFP itself, were C-terminally fused to GFP.
Cells were cultured in LB medium and whole cell fluorescence was measured 8 hours after induction with 0.4 mM IPTG. For protein production in Lemo21(DE3), the optimal rhamnose concentration was used. Notably, fluorescence values of TspA and TspB were multiplied by 10, and fluorescence values of GST-GFP and GFP were divided by 10 and 50, respectively.
In Figure 2C, an example of the optimization of the production of a disulfide bonded single-chain variable fragment (scFv) in the periplasm of Lemo21(DE3) is shown. To guide the scFv to the periplasm it was equipped with a signal sequence (p = precursor protein, localized in the cytoplasm), which is clipped off upon translocation of the protein into the periplasm (m = mature protein, localized in the periplasm).
Notably, the scFv produced at the optimal rhamnose concentration was functional.
Taken together, the versatility of Lemo21(DE3) makes it possible to rapidly identify the optimal conditions for the production of any protein using one strain and a limited number of culture/induction conditions.
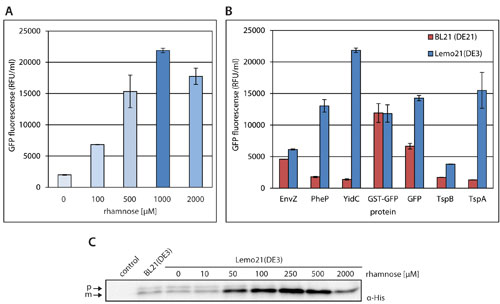
Figure 2. Optimizing protein production using the Lemo21(DE3) strain
Rhamex System
The rhamnose promoter system that is used to drive the expression of the gene encoding the T7 lysozyme in the Lemo21(DE3) strain can also be used directly to drive the expression of genes encoding target proteins. The Rhamex system offers tight control over protein production. The rhaR and rhaS regulatory genes as well as the rhaBAD promoter have been cloned into a series of vectors with different copy numbers, resistance markers and multiple cloning sites (Figure 3A).
The Rhamex system makes it possible to control the target gene expression levels in an unprecedented manner. This greatly facilitates optimizing the production of both routine and difficult proteins in any E. coli strain and other Gram-negative bacteria.
Recently, Xbrane Bioscience has developed several proprietary E. coli strains for the Rhamex system. In these strains protein production levels can be reached that are considerably higher compared to regular strains (Figure 3B). On request, an antibiotic-free selection setting can be created for the Rhamex system.
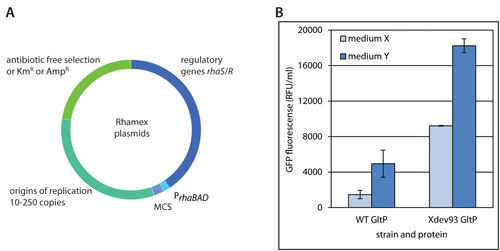
Figure 3. Set-up of the Xbrane Rhamex System and comparison between wild type (WT) cells and a proprietary Xbrane Rhamex Strain (Xdev93) producing the polytopic membrane protein GltP C-terminally fused to GFP in different culture media
David Vikström, Ph.D., is a scientist at Xbrane Biosciences. Susan Schlegel is finishing her Ph.D. studies at Stockholm University. Jan-Willem DeGier, Ph.D. ([email protected]), is CSO at Xbrane and an associate professor at Stockholm University.



