January 1, 2016 (Vol. 36, No. 1)
Angelo DePalma Ph.D. Writer GEN
Transient Transfection Can Reward Impatience by Quickly Providing High Protein Titers without Penalizing Production Down the Line
Transient transfection has become the go-to method for obtaining gram quantities of protein for characterization and early-stage preclinical studies, without having to wait for stable transfection and clone selection.
“The key to a successful utilization of transient transfection during early phases is to mirror cellular conditions to conditions expected to be used in final production,” says Daniel Ivansson, staff research engineer, strategic technologies, GE Healthcare. That is, use the same cell line for transient transfection and production.
Transient transfection has its complications. For example, certain cell lines, such as CHO cell lines, can be difficult to transfect. Fortunately, stubborn cell lines often become more pliant with the application of MaxCyte flow electroporation technology.
Another complication, perhaps as serious, is the need to accomplish transfection with an adequate degree of control. Here, control means ensuring that mRNA levels, and hence the expression loads for the proteins of interest, are similar to those that one would expect to find in a stable cell line during process conditions.
“That’s difficult to achieve with plasmid DNA,” Ivansson notes. To keep mRNA levels within bounds, he suggests the alternative of using in vitro synthesized mRNA instead of using plasmid DNA for transient transfection: “mRNA is potentially easier to titrate, which should improve control.”
Ivansson says that electroporation allows good control over the first step of getting nucleic acid into the cell, but does not help to control intracellular events, for example, entry of plasmid DNA into the nucleus.
“The plasmid must be transcribed into mRNA, which must be retransported into the cytoplasm,” explains Ivansson. “Because these steps are hard to control, the entire process becomes difficult to regulate, and that affects the quantities of final mRNA in the cytosol and hence the expression load.”
Because transfection with in vitro synthesized mRNA involves only one step—getting the mRNA into the cytosol—the overall process is under much tighter regulation.
Transient transfection technologies promise decently high protein titers in about one to two weeks, as opposed to several months, for cells from stable gene integration. Or so goes conventional wisdom.
But there is an alternative. Instead of going through stable transfection, clone selection, and expansion, one can use pools of stably integrated cells instead. With current state-of-the-art CHO expression platforms, titers sufficiently high for preclinical work (0.5–1 g/L) can be achieved from stable pools within five to eight weeks. For platforms using site-directed integration of the gene of interest, the time to stable pools should be reduced even further.
“This is a simpler, more straightforward way to screen many different constructs using conditions predictive to final process conditions,” Ivansson opines. “I’m not sure anyone really needs transient transfection, at least not for material required for early-stage work.”
Through its partnership with Promosome, GE Healthcare is currently developing a platform methodology based on site-directed integration of a single copy of the gene of interest. This technology should enable gram-level production from stable pools within a timeframe similar to transient transfection and with the added benefit of predictive cellular conditions and a straightforward path to final production clones.
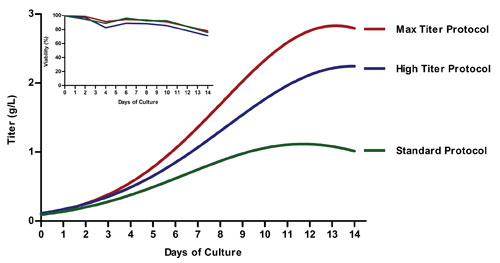
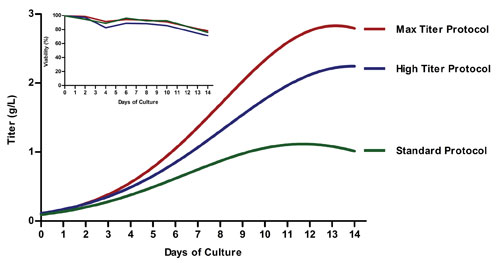
Human IgG expression for the Standard (green), High Titer (blue), and Max Titer (red) ExpiCHO expression protocols from Thermo Fisher Scientific. The main graph gives kinetics information. Standard: one feed, no temperature shift. High Titer: one feed, a temperature shift to 32°C. Max Titer: two feeds, a temperature shift to 32°C. The inset gives information viability. Standard: viable cell density post-transfection. High Titer: viability post-transfection for ideal expression run. Max Titer: viability post-transfection for troubleshooting.
Translational Applications
MaxCyte’s flow electroporation technology was originally designed for human primary cells, as part of cell therapy. “Our design criteria were quite high because we needed to process a sufficient number of cells rapidly, efficiently, safely, reproducibly and at clinical scale,” says Doug Doerfler, MaxCyte’s CEO. Eventually, scientists began inquiring about flow electroporation for research purposes. The rest is history.
Top pharmaceutical and biopharmaceutical companies now use MaxCyte’s technology to engineer cells for research and screening applications, plus rapid production of vaccines and proteins. The company retains its strong roots in cell therapy, while continuing to support advanced research and development applications.
“We’re seeing a lot of translation between research and therapy applications,” Doerfler informs. “The most prominent examples are gene editing with CRISPR, zinc finger nuclease, and other systems where the same core technology that is used for therapy is applied to biopharmaceutical production. We’re also seeing exciting applications in CHO cells, where the scalability, speed, and efficiency of flow electroporation begs the question of what else can be achieved with these cells.”
Doerfler refers to the “democratization” of gene editing and transient transfection, whereby companies that used to outsource cell-engineering work now try it in-house. This leads to companies moving toward using these technologies to develop novel cell lines in-house vs. using services or “closed-architecture production systems” that require utilization of a specific combination of cell lines, vectors, and reagents.
Ultimately, transient transfection and cell-line engineering will enable the development and manufacture of new classes of therapeutic proteins that could not be expressed before.
“Developing these rapidly, through transient transfection, will reduce development bottlenecks and expand the number of variants you look for, which will result in safer, more effective drugs,” Doerfler tells GEN. “It also creates options for the types of cells you can use to produce those proteins.
“What has been obvious for primary cells is now possible for mammalian and insect cells. Eventually advances in cell engineering technologies—and not just ours—will become routine in drug development.”
The Trouble with CHO
Why are CHO cells difficult to transfect? “The ability of a cell to take in and express exogenous DNA depends on cell type,” notes Laura Juckem, Ph.D., R&D group leader, Mirus Bio. “For largely unknown reasons, HEK293 cells are very easy to transfect, perhaps because of their cell surface receptor profile, cell division rate, or other factors. The same rationale can be used to explain why CHO cells are more difficult to transfect.”
Transient transfection of CHO has improved over the years. One strategy involves generating engineered CHO cell lines that maintain transfected plasmids episomally, which provides transgene expression over time. The insertion of cis elements into the expression vector backbone enhances episomal maintenance of a transfected plasmid.
Other high-yielding transient transfection methods have been reported, for example, with high-density cultures, dimethyl sulfoxide pretreatments, and low-temperature post-transfection conditions. Dr. Juckem notes that the adaption of normally adherent CHO-K1 cells has also achieved limited success in large-scale transient transfection.
Her group at Mirus Bio has reported on a CHO-S platform that produces approximately 375 mg/L of antibodies 10 days after transfection, levels comparable with HEK293-FS cell expression.
CHO-K1 is the original lineage isolated in 1957 by T.T. Puck. Another variant, CHO-S, is also commonly used for transient transfection.
Dr. Juckem admits that these titers are modest compared with stably transfected cell lines: “But here, for transient transfection, the gene of interest is not integrated into the genome. Therefore, expression is highly dependent on the protein of interest, plasmid, culture medium, and other experimental parameters.”
The Media Are the Message
In October 2015, Irvine Scientific introduced BalanCD® Transfectory™ CHO, a cell-culture medium for rapid and scalable transient transfection of CHO cells. BalanCD is chemically defined and animal component free. It was designed to support increased transfection efficiency and gram-scale protein expression.
Obtaining gram-scale yields through transient transfection in CHO cells is expected to cut the costs and time for biopharmaceutical development. At the same time, using the same organism during development and production helps maintain protein quality throughout the product’s lifecycle, thus enabling scientists to produce the proteins needed to assess candidates using rapid and cost-effective methods, according to an Irvine press release.
“People have been using HEK293 cells for transfection because existing technologies did not yield sufficient protein quantities in CHO cells,” says Jessie Ni, Ph.D., CSO at Irvine.
While developing its new medium, Irvine worked with the National Canadian Research Council and used its polyethyleneimine (PEI)-mediated transfection methodology in combination with the improved medium. Dr. Ni asserts that “the optimized medium and protocol resulted in about a two- to fourfold improvement in transient gene expression,” and that biomanufacturers “can now use CHO for their development work, which provides process consistency and facilitates transition into production.”
Aside from electroporation, transient transfection in CHO was traditionally limited to yields of under 1 g/L. For their new media, Dr. Ni and colleagues aimed at a typical titer obtained from HEK293 transient transfection, approximately 1 g/L. “That was our benchmark,” he insists.
Irvine says that using its PEI-mediated transfection protocol in combination with the new BalanCD product is simple and scalable. It involves expanding CHO cells over a few days, transfection without the need for media exchange, and the addition of post-transfection supplements and temperature shifts to enhance titer.
“The media improvement changes cell growth behavior to support sustained growth and viability,” Dr. Ni explains, “so as a result you also must optimize transfection parameters, such as the cell density, the timing of transfection, and the ratio/amount of PEI and DNA.”
Media optimization can achieve even higher titers. In September 2015, Thermo Fisher Scientific introduced the Gibco ExpiCHO™ Expression System. According to the company, this systems enables protein yields as high as 3 g/L.
“Development of the product was based on a strategy similar to that of our Expi293™ transient expression system, which was launched three years ago,” says Henry Chiou, Ph.D., associate director of product management at Thermo Fisher.
“We wanted to achieve yields from our CHO system that were equivalent or higher than what can be obtained from the best-performing HEK293-based systems,” he continued. “That meant redesigning everything—culture media, transfection reagent, transfection enhancer, a feed for the culture, and the CHO cells themselves—so that all would synergistically work together to increase expression.”
One result of optimization was to increase culture density at the time of transfection from the typical 1 million cells/mL to 6 million cells/mL. CHO cells were further optimized for yield through clonal selection. Dr. Chiou calls this strategy a “one-two punch” toward improving volumetric productivity.
Moreover, the medium was enriched and matched to a feed supplement to provide nearly two weeks of viability and expression, compared to one week for standard transient transfection systems. Together, these improvements led to increases in protein expression of as much as a 100-fold compared with conventional reagent-based transient CHO expression systems.
Initially, ExpiCHO was evaluated in-house on a small set of reference proteins. Additional beta testing at three dozen customer sites, on various other proteins, confirmed that expression levels in ExpiCHO were consistently two to three times higher than those for Thermo Fisher’s Expi293 product and other 293-based systems. “Transient transfection in CHO can now generate more protein, and it becomes more cost-effective than in 293 cells,” Dr. Chiou observes.