June 15, 2013 (Vol. 33, No. 12)
Overcoming toxicity and poor accumulation are common themes in many unsuccessful attempts to overexpress difficult proteins such as multispanning membrane proteins.
According to Genentech’s Hok Seon Kim, Ph.D., senior research associate in antibody engineering, toxicity can be suppressed and cell growth improved to normal by tightly controlling transcription.
“In addition, translation initiation rates play a critical role in proper targeting, insertion, folding, and efficient accumulation of integral membrane proteins in the E. coli membrane,” Dr. Kim said. The protein candidates for expression evaluations are not themselves used in therapy, but serve as potential targets for other therapeutic drugs.
Toxicity to the expressing organism is a problem with many recombinant proteins, especially as the titer builds up. In the case of the transmembrane proteins, Dr. Kim observed toxicity as early as the cloning stage, in the form of restricted or inhibited cell growth.
Many transmembrane proteins appear to be more toxic than, say, antibodies. “It’s thought that their toxicity results from disruption of the biogenesis of endogenous membrane proteins,” Dr. Kim explained.
In E. coli, the availability of native molecular machineries for membrane protein biogenesis is limiting, particularly the protein that recognizes the first transmembrane domain of multitransmembrane proteins.
“This molecule, SRP, must catch that first hydrophobic transmembrane domain as quickly as possible and target it to the membrane with the help of its receptor,” Dr. Kim said. “That particular signal recognition particle (SRP) is limiting in E. coli.”
In other words, during overexpression of transmembrane proteins, E. coli’s endogenous membrane proteins must compete for the limiting SRP to maintain homeostasis. An expression construct with a too strong translation initiation rate would result in a quick buildup of ribosome-nascent chain complexes with missing signal recognition particles and continue to translate in the cytoplasm, resulting in degradation and/or aggregation. Delayed availability of the SRP results in improper targeting and/or insertion, and re-routing to pathways associated with degradation and/or aggregation.
Poor accumulation is the consequence of this constant high level of degradation and/or aggregation in conjunction with inefficient membrane targeting. The traffic jamming of the membrane protein biogenesis pathway results in “sick” cells.
“It is even worse with GPCRs,” Dr. Kim said, “but there may be other factors at work there, that make some proteins more toxic than others among the class of membrane proteins.”
Dr. Kim’s improvement involves a novel promoter with “tight” transcription regulation to minimize toxicity and improve cell growth. Because toxicity and growth inhibition are observed even before induction, expression efforts begin with compromised cells “and the problem gets worse towards the end,” he said.
In addition, Dr. Kim has used a translational leader to control the translation initiation rate. An optimal translation initiation rate would eliminate the traffic jam and allow efficient recycling of the cell’s molecular machineries, resulting in continuous production of heterologous and endogenous membrane proteins.
At Manufacturing Scale
Expressing difficult proteins is most challenging in a production setting, where the manufacturability of stable, correctly folded proteins often determines success or failure.
Speaking at the “PEGS” event this April, Ian Hunt, Ph.D., head of protein sciences at the Novartis Institute for BioMedical Research, hashed out the pros and cons of E. coli and baculovirus expression as it relates to difficult-to-produce proteins.
Baculovirus expression requires insertion of DNA into a viral genome, transfection, and two or three rounds of amplification to produce enough virus at the correct titer.
“With insect cells, it typically takes two to three weeks before you can determine if your protein is expressing,” Dr. Hunt said.
E. coli is a more efficient and accessible system than insect cells, in part because it does not involve a viral component. Cells are transformed directly and begin expressing protein, of which measurable quantities become available within a few days, sometimes overnight.
“E. coli is much faster, and much more amenable to high-throughput expression testing,” Dr. Hunt said. It is possible to fragment very difficult protein targets into 10 or 20 smaller domains, express them in parallel in E. coli, and see which is the most stable and prolific. “It doesn’t take much time, and does not require a lot of hands-on time. But the same process is quite laborious in insect cells,” he added.
Dr. Hunt described the relative benefits and capabilities of the two expression systems as a tradeoff.
“E. coli is fast and easy, but it’s most applicable for relatively small, easy to produce proteins, whereas insect cells can produce larger, difficult proteins but it takes a lot longer.”
Insect cells are capable of expressing large, difficult, multidomain, intact proteins that E. coli cannot. Once a protein reaches 60–70 kD in size, its soluble expression becomes problematic in E. coli.
Increasingly, pharmaceutical and biotech companies are turning to difficult protein targets that, more often than not, are difficult-to-express membrane proteins. Breaking those proteins up works to produce small, easy-to-manufacture subdomains as targets for drug discovery.
Another topical target of pharmaceutical development is disruption of protein complexes. One way to obtain complexes is to express and purify the relevant proteins individually. Dr. Hunt described an approach, co-expression, by which two or more viruses transfect insect cells simultaneously, resulting in the production of an equivalent number of proteins within the same insect cell culture.
After simultaneous purification, the proteins are in the proper proximity for complexation. Disruption might occur through direct inhibition of binding sites or by allosteric interactions. Or, one could label one protein with an affinity tag, purify the complex as one “molecule,” and use it in drug screens.
“Co-expression is one of the major strengths of insect cells,” Dr. Hunt observed.
Stabilizing Disulfide Bridges
Elsewhere at the PEGS event, Alan Dombkowski, Ph.D., assistant professor of clinical pharmacology and toxicology at Wayne State University School of Medicine, discussed techniques for enhancing the thermal stability of proteins through disulfide bond engineering.
He has developed a software package, Software By Design (DbD), that facilitates the rational design of disulfide bonds in proteins.
Nature uses disulfide bonds to stabilize proteins, particularly small secreted proteins that lack a stabilizing hydrophobic core. DbD locates amino acids that are candidates for site-directed mutagenesis that transforms these locations to cysteines, which are then primed for disulfide bond formation. Both intramolecular and intermolecular disulfides are possible, but the latter are more difficult to achieve.
Dr. Dombkowski noted a recent publication in which investigators enhanced the thermal stability of lipase B, which already has a disulfide, by introducing a second sulfur-sulfur linkage. Higher thermal stability provided a more robust process, in this case, for manufacturing biodiesel.
“In most industrial processes, the higher temperatures they can run the reactions at, the better,” Dr. Dombkowski observed. A recent patent application, by DbD licensee Novo Nordisk, describes introduction of a disulfide linkage in growth hormone to make the molecule more resistant to proteolytic degradation. In some cases, disulfide formation improves activity as well, but this is where care must be taken. In a third example Dr. Dombkowski relays, an antibody’s thermal properties were significantly enhanced but activity fell. “The potential effects on activity are real,” he said.
DbD begins with a protein’s structure, and suggest locations where amino acid switches to cysteine are likely to produce a disulfide bridge successfully. The locations must be relatively close in space, but the residues must also possess the correct angle and orientation.
DbD was developed by examining naturally occurring disulfides and characterizing their atomic coordinates, orientations, and geometric requirements, and extrapolating from there to the putative target protein protein. “The software is quite good at modeling these systems, and predicting if the disulfide bond will form,” Dr. Dombroski remarked.
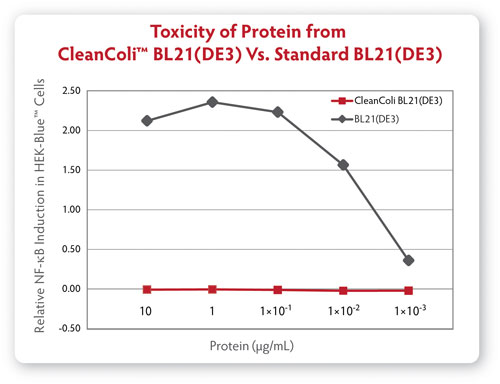
Meantime, Curtis Knox, marketing director at Lucigen, discussed what could be a game-changer for E. coli as an expression system. Available since May, his firm’s CleanColi™ competent cell E. coli strain uses genetically modified lipopolysaccharide (LPS) that does not cause an endotoxic response in humans. Endotoxin removal has been one barrier to employing E. coli as protein expression systems, and using E. coli-derived proteins in cell-based assays.
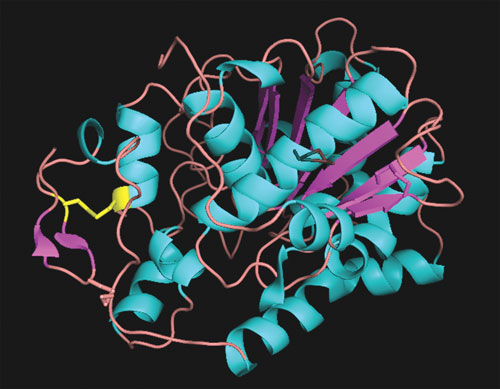
An engineered disulfide bond (yellow) provides a significant increase to the thermostability of Candida antarctica lipase B (CalB), an important industrial enzyme. Computational analysis with Disulfide by Design and other software revealed prospective sites for creating novel disulfide bonds within CalB. The disulfide bond providing the greatest improvement in thermal stability (8.5°C) is shown. This work is described in Le et al., Biotechnology and Bioengineering, 2012 Apr;109(4). [Wayne State University]
Endotoxin-Free E. Coli
“E. coli is commonly used for research-scale protein expression but its ease of use has been limited by unwanted endotoxin contamination. These days are hopefully over,” said David Mead, Ph.D., Lucigen founder and CEO.
CleanColi is available as a research-only product, but Lucigen expects that to change. “We anticipate that manufacturing companies may want to investigate these cells for bioproduction, and we will certainly work with them on that,” said Knox. “We believe CleanColi holds that potential, but it has not yet been scaled to that level.
How can E. coli survive without the naturally occurring lipopolysaccharides that comprise their outer membrane? Lucigen has incorporated genetic deletions that alter the lipopolysaccharide into a different molecule, Lipid IVA, which allows the cells to thrive and express protein, without generating harmful endotoxins.
“Processes that avoid E. coli could now consider that expression system,” Knox said. “Many bioprocessors have shied away from E. coli, even though it’s a very easy system to work with, and easily scaled, but have not due to cost and time and yield loss associated with endotoxin removal.”
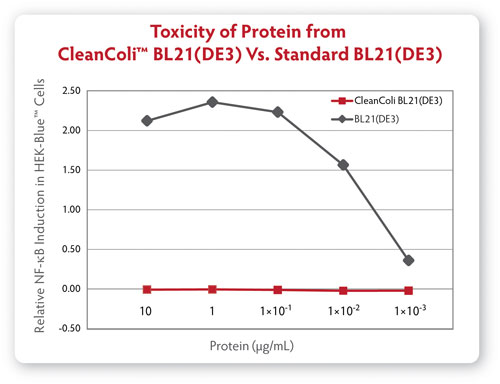
Comparison of endotoxic response from protein derived from Lucigen’s CleanColi BL21(DE3) and traditional BL21(DE3) competent cells.
Codon Optimization
Given that organisms have 61 codons at their disposal (plus 3 stop codons), but just 20 amino acids, organisms can “select” among 3 codons for each amino acid. The preference of an expression system for particular distribution of synonymous codons, known as codon selection bias, has no effect on a protein’s primary structure but may affect expression level or yield. Controlling codon bias is one of the technologies offered by DNA2.0 for improving protein expression and yield.
Codon optimization is not strictly about selecting the “best” codon, but the most productive balance or distribution of codons throughout the protein.
“We’re trying to optimize gene performance, meaning protein output,” explained Mark Welch, Ph.D., DNA2.0 director, R&D. “This is a very big deal. Some drugs never leave the development stage because their expression is insufficient for running clinical trials.”
Among these “problem” proteins are difficult-to-express membrane proteins. Optimizing codon selection may improve expression and yield as much as 10-fold. In some situations even a small percentage increase may be worth the effort involved in redesigning a gene.
Most of the impact of codon usage optimization occurs at the RNA level, in particular in the sequences, the triplets employed to code for individual amino acids. Some effects may result from the RNA’s local structure. For example, how RNA folds may affect whether ribosomes can locate the message and translate it into protein. Another factor is stability.
“Many natural RNAs have programmed lifespans,” Dr. Welch said. “In nature, some RNAs are present at high levels for a very short time, and it is possible to build in degradation sites that limit its lifespan.” But for production systems high levels of highly stable RNA are preferred.
“You’d want to avoid sites that lead to high degradation,” he added. Other regulatory motifs that come into play include processing sites. “We consider many things in the design, which may be affected when we re-code genes,” Dr. Welch said.