September 15, 2013 (Vol. 33, No. 16)
Vicki Glaser Writer GEN
To predict drug response or toxicity, the pharmaceutical industry is increasingly performing smaller-scale validation studies of experimental drug compounds in novel 3D cell culture models intended to mimic more closely the structure, activity, and extracellular environment of tissues in vivo.
Following high-throughput, large-scale screening assays performed in 2D systems, these smaller, secondary screens in 3D microtissue models are more likely to be predictive of how cells will react in vivo. Several presentations at SMi Group’s recent “Cell Culture” conference in London focused on advances in novel 3D cell culture systems and applications.
The things we see in 2D may not reflect what is happening in vivo, mainly due to the “lack of architecture in 2D systems, which influences the biological response in many difference phenotypes,” said Olivier Pardo, Ph.D., team leader at the Cellular Regulatory Networks Group, Imperial College, London. One clear example he cited is the response of tumor cells to drug therapy and the fact that tumor cells grown in 2D versus 3D cultures tend to respond quite differently to the same concentration of an antitumor drug. Tumor cells grown in 3D are typically more resistant to therapeutic compounds.
When cells grow in 3D spherical clusters, compared to 2D monolayers, the level of oxygenation differs depending on the whether a cell is situated more toward the inside or the outside of a spheroid structure. The difference in level of oxygenation appears to influence the response to drug therapy, explained Dr. Pardo. Additionally, differences in the extracellular matrix produced by cells growing in 3D culture systems can modify the cellular response to drugs and other stimuli, likely because of changes in integrin-based signaling in 3D compared to 2D cell cultures.
Another factor that can affect the therapeutic drug response of cells grown in culture is the extent of cell-to-cell contacts, which will tend to be more developed in 3D environments with higher cell densities.
3D culture systems are especially useful for studying the invasive properties and metastatic potential of tumor cells and for conducting screening assays for cell migration. In his presentation, Dr. Pardo described screens of large compound libraries using a bone metastatis assay or extra/intravasation assay, small-to-medium library screens with a 3D collagen invasion assay, and screening of a small number of targets using a zebrafish metastatic model organism. The 3D collagen invasion assay with confocal image acquisition was performed in 96-microwell plates.
Whereas manual screening was slow and difficult, making this assay format ill-suited for large library screening, the results obtained were highly reproducible, with >90% overlap between repeat results. In contrast, automating each step of the assay—and providing temperature control to minimize collagen polymerization—increased the throughput of the assay to make it suitable for large-scale screening, but the reproducibility of the robotized screen was poor, with <33% overlap between repeat results.
Limitations related to automation and the cost of most 3D cell culture technologies and materials available today are two important factors that stand in the way of using these methods for large-scale screening campaigns and of more widespread adoption of the technology, in Dr. Pardo’s view.
One of the main advantages of the 3D collagen assay and of 3D culture systems in general is the ability to do co-cultures, noted Dr. Pardo, thereby more closely mimicking the in vivo environment and enabling the introduction of cells capable of producing growth factors and other natural molecules to support the health and viability of the cultured cells.
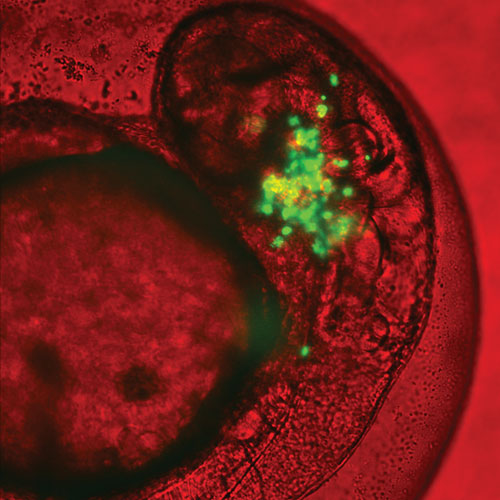
Olivier Pardo and colleagues at the Cellular Regulatory Networks Group, Imperial College, London, use a combination of 2D and 3D cell culture assays and whole zebrafish models to study the invasiveness and metastatic potential of tumor cells. This image depicts green fluorescent protein (GFP)-labeled lung cancer cells growing in a zebrafish embryo, a model system used to study metastasis in vivo.
An Automatable Liquid Scaffold System
At Trinity College in Dublin, Anthony Davies, Ph.D., director of the high content facility, and colleagues developed a novel liquid scaffold system that permits 3D cell culture and subsequent harvesting of the cells. The researchers designed the system to be compatible with essentially any automated liquid-handling and high-throughput screening system, as well as high-content screening and image-analysis technology.
“Unlike any other 3D assay systems currently used, our technology does not rely upon solid gel matrices, scaffolds, micropatterned surfaces, or hanging-drop assay systems to achieve reproducible cancer spheroid growth,” said Dr. Davies. “Indeed, many of the inherent technical issues surrounding these technologies are avoided using our novel 3D culture technology.”
He described the innovative liquid scaffold, the ease of doing automated 3D cell culture, and the ability to perform subsequent analyses on the isolated cultured multicellular constructs—for example, by applying biochemical, imaging, and Raman assay technologies.
This polymer-based scaffold system has the properties of a gel, yet it remains a liquid and does not solidify. The liquid has the same low viscosity, density, and properties of a regular cell culture media and is appropriate for growing virtually any cell type, according to Dr. Davies. The modified, natural polymers in the system form an elastic, reversible scaffold. Cells added to the liquid do not sink. They are able to form spherical multicellular constructs and other 3D structures. The cultured cells can be labeled, stained, exposed to experimental drugs, evaluated for their responsiveness, and essentially studied as one would cells grown in a conventional suspension culture. Addition of a deactivating agent disrupts the polymers, releasing the 3D cell structures for harvesting.
The researchers have produced this material in microliter to liter volumes. They have outlicensed the technology to Biocroi, which markets the product as Happy Cell® ASM (advanced suspension media).
Studying Drug Response in 3D Systems
The literature contains many examples of studies demonstrating differences in drug response in 3D models for monolayer systems. For instance, in a paper that appeared July 18 in the online version of Biochimica et Biophysica Acta, General Subjects, the authors (H.J. Mulhall et al.) evaluated the different electrical properties of epithelial cancer cells cultured in 2D and 3D environments and concluded that “factors such as cell shape and cytoplasmic trafficking between cells play an important role in their electrophysiology,” highlighting “the need to use in vitro models more representative of native tissue when studying cell electrophysiological properties.”
The advantages of a label-free monitoring system derived of human embryonic stem cell-derived cardiomyocyte clusters for predictive in vitro cardiotoxicity testing includes a more representative tissue milieu than traditional monolayer cell culture, as indicated in a paper that appeared July 8 in PLoS One. The authors (H.-G. Jahnke et al.) described how they monitored the adverse effects of drug exposure for more than 35 days while the clusters retained their structural and electrophysiological characteristics. The system “provides multiparameter analysis capabilities incorporating field potential recording, over days or even weeks,” said the authors.
The aim is to grow cells in 3D in vitro culture systems that mimic as closely as possible in vivo cellular microenvironments, including the effects of cell positioning within 3D constructs, cell-to-cell contacts, the extracellular matrix, cell signaling, and the factors associated with co-culture of multiple cell types. These same factors are as important in studying drug responsiveness in cultured microtissue constructs, as in evaluating the efficacy of therapeutic strategies targeting tumors.
In the latter case, the ability to simulate the microtumor environment, communication between tumor cells, the effects of the tumor stroma environment, and the interaction of tumor cells with other surrounding cell types such as epithelial cells or fibroblasts, can enhance the ability to study mechanisms of tumorigenesis, cell migration and invasion, and metastasis.
One of the main and ongoing challenges has been to develop in vitro 3D cell culture systems that are compatible with industrial-scale applications and are readily automatable for high-throughput screening assays.
Jens Kelm, Ph.D., CSO and co-founder of InSphero, described three 3D cell culture methods that are currently most broadly accepted in the industry: cell constructs embedded in hydrogels; cells grown in scaffolds; and cellular self-assembly leading to spheroid formation. InSphero’s approach leads to the formation of scaffold-free 3D multicellular spheroids in a 96-well format. The company’s GravityPLUS™ technology automates the classic hanging drop methodology, in which cells in hanging drops of culture media descend and assemble into microtissue spheroids without the need for any support matrices or contact with any surfaces.
“We have exploited the versatility of hanging drop production by uncoupling the generation of microtissues from downstream applications,” said Dr. Kelm. “This was the creation of our platform, producing very uniform spheroids or microtissues while also allowing for compound treatments, microscopic analysis and assays, to get the best of both worlds!”
In his presentation at the SMi meeting, Dr. Kelm described the availability of an increasing variety of model systems available to industry and the research community for testing the safety, toxicity, and efficacy of drug compounds in in vitro systems and minimizing the need for animal testing. The demand for good in vitro model systems for screening extends beyond the pharmaceutical industry, with safety assessment of chemicals and other compounds important for the chemicals and cosmetics industries as well.
“We are using reporter systems that are allowing us to perform target validation in 3D model systems,” said Dr. Kelm. This has revealed sometimes substantial differences in the effects of drugs on microtissues versus cells grown in monolayer systems, and in particular in models of tumor growth and proliferation.
One goal going forward is standardization. It would be convenient, for example, to have standardized 3D liver and cardiac model systems available for safety testing. “It is important to generate comparable results, to test on the same model and be able to compare results over years and across drugs,” added Dr. Kelm.
Dr. Kelm foresees continued progress in mimicking interconnected, complex tissue constructs in functional living tissue models—so-called body-on-a-chip concepts. Two consortia, one in the United States and one in Europe, are working on this concept. InSphero is participating in the European project, in which the different tissue types are produced externally as 3D spherical constructs and then loaded onto a microchip, which is composed of interconnected compartments.
“There is consensus in the pharmaceutical industry that 3D model systems can provide a higher quality of information in vitro,” concluded Dr. Kelm.
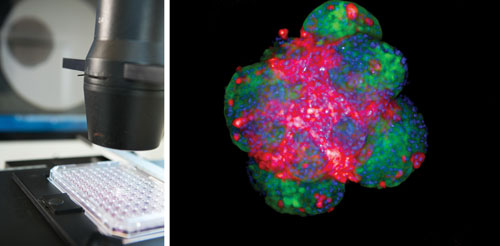
96-well microtissue assay plate (InSphero’s GravityTRAP™) loaded with tumor microtissues composed of the colon cancer cell line HCT116 (left) expressing a green fluorescent protein and stromal mouse fibroblasts NIH3T3 expressing a red fluorescent protein. Visualization of the individual cell populations using the PerkinElmer Opera QEHS equipped with a 10x Air objective. The single z-stack through the microtissue was processed with the Acapella High Content Imaging and Analysis Software to generate a maximum projection image (right). [Image provided by PerkinElmer, Karin Boettcher and Stefan Letzsch]
Nanofibers
Peptisyntha, a member of the Solvay group, applied its expertise in peptide synthesis chemistry to develop a family of short peptides that are able to self-assemble and form nanofibers. These nanofibers exist in a hydrogel capable of supporting 3D cell cultures.
“The major benefit achieved with 3D or pseudo-3D culture is the higher cell density that can be obtained when compared to 2D culture,” said Marc Fouassier, business development manager. “This is an extremely important aspect for the manufacture of certain cell lines, and certain targeted cell applications for instance in cell therapy.”
Using Peptisyntha’s hydrogel system, cells grow on a soft coating prepared from the self-assembling peptides, which mimics the extracellular matrix. The peptide coatings can be prepared on various plastic surfaces traditionally used for cell culture, including polystyrene, polyethylene terephthalate, and polycarbonate.
According to Fouassier, advantages include the fully synthetic, animal-free, GMP origin of these hydrogel peptides, which are particularly useful for culturing cells that require adhesion.
Human Brain Tissue Grown in Test Tubes
Researchers from the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences report the development of human brain tissue in a 3D cell-culture system. Their technique, which is discussed in an article last month in Nature (“Cerebral organoids model human brain development and microcephaly”), permits pluripotent stem cells to develop into cerebral organoids, or “mini brains,” that consist of several discrete brain regions.
“Our goal was to create a model system of the human brain,” said Jürgen Knoblich, Ph.D., IMBA’s deputy scientific director during a press conference.
Intrinsic cues from the stem cells guided the development toward different interdependent brain tissues. Using the mini brains, the scientists were able to model the development of a human neuronal disorder (i.e., microcephaly) and identify its origin.
“A normal developing brain has a stem cell population that undergoes rounds of division at specific times to make more stem cells and eventually neurons,” noted Dr. Lancaster. “But the microcephalic patient-derived stem cells made neurons too early in the process. This led to a depletion of the stem cell population, which resulted in fewer neurons being made.”
Putting it another way, Dr. Knoblich explained that this finding led to the hypothesis that, during brain development of patients with microcephaly, the neural differentiation happens prematurely at the expense of stem and progenitor cells, which would otherwise contribute to a more pronounced growth in brain size. “Further experiments also revealed that a change in the direction in which the stem cells divide might be causal for the disorder,” he continued.
The new method offers great potential for establishing model systems for human brain disorders, according to the researchers. Such models are urgently needed, as the commonly used animal models are of considerably lower complexity and often do not adequately recapitulate the human disease.







