June 15, 2013 (Vol. 33, No. 12)
Silicon Microchip Capable of High-Frequency Fluidic Valving at Heart of Technology
The purification of cellular populations and individual cells is pivotal for the reliable characterization of gene expression, many aspects of life science research, and the development of cellular therapies. Cell sorting routinely involves fluorescence-based separation through flow cytometry, which has proven superior to crude techniques such as differential sedimentation. Through this process, large numbers of cells are rapidly analyzed for specific fluorescence signatures. Traditionally, cell samples are parsed in charged aerosol droplets, which are electrostatically sorted, enabling purification at thousands of cells per second at purities often greater than 90%.
This technology enables exceptional specificity using multiple fluorescent signatures (e.g., cell surface labels, cell size, and granularity).
However, the contributions of current flow-sorting platforms are balanced against significant limitations, including: high processing pressures that can result in loss of function and/or cell death; sample processing speeds/volumes that make processing clinical-scale samples (>500 million cells) unfeasible; a high degree of technical expertise needed to manage device complexity; increased risk of sample contamination through the use of open systems; and user safety concerns when processing aerosolized patient samples.
These limitations, plus the high unit and sample processing costs, must be overcome to enable further clinical application and commercialization.
To address these issues, Owl biomedical developed a fully closed cell-sorting system. This microchip-based technology employs closed fluid path cartridges with aseptic ports that permit the straightforward administration and collection of cell samples. At the heart of the cartridge is a patented microchip capable of very high-frequency fluidic valving (Figure 1).
Propelled by modest positive pressure, typically less than 0.2 atmospheres, cells pass through microchannels where laser-directed fluorescent signals are detected with photo-multiplier tubes. Upon identification of a positive target cell, the microchip valve opens, redirecting the cell to a collection chamber. Both positive and negative selected cells can then be retrieved from the cartridge and used for any number of downstream applications.
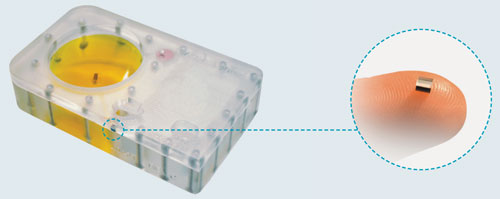
Figure 1. Microchip and microchip-based sorting cartridge
Improving Valve Speed
One key design goal of all cell sorters is to maximize the speed at which the device can segment a stream of cells. In the case of Owl’s microchip-based technology, a fluidic valve determines the rate at which cells are isolated. Valve speed in a fluid microenvironment is in turn controlled by several factors, including the acceleration and magnitude of the opening and closing forces, and the inertia of the valve and the fluid surrounding it.
In the case of Owl technology, valve speed is controlled by its engineered magnetic properties and a powerful return-spring force, which serves to close the valve (Figure 2). Careful modeling and empirical testing has led to a design that allows μsec opening times, a user-selected sort collection delay, and μsec closing times.
A typical total cycle of around 50 μsec allows separation rates similar to a traditional droplet sorter, although with microchip-based sorting no aerosols or droplets are used.
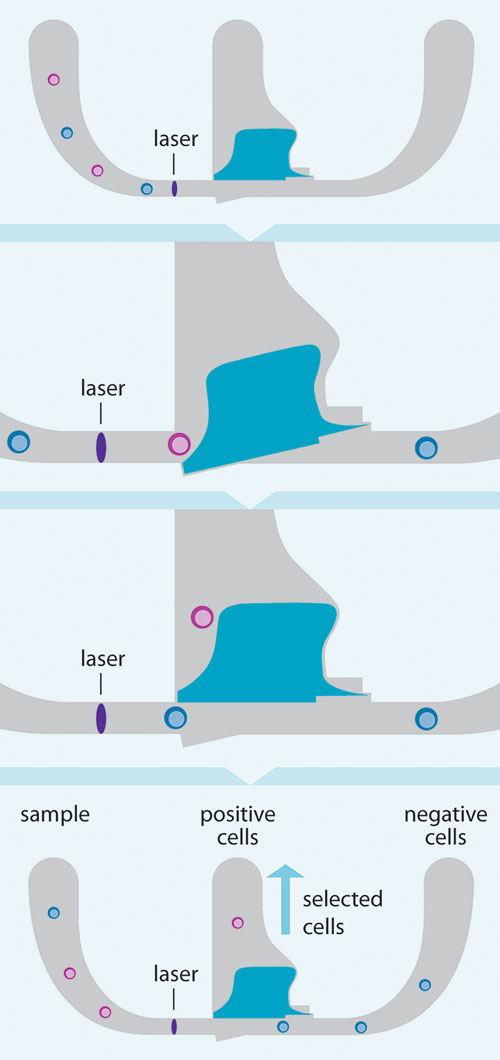
Figure 2. Mechanism of microchip-based sorting: Labeled cell samples enter the chip from the input sample, as the cells approach the sort area each cell is analyzed. When a selected cell is identified a magnetic pulse opens and closes the valve and the cell is redirected to a collection chamber. An integrated single-crystal silicon spring returns the valve to its original position, and undesired cells are allowed to flow through.
Highly Viable Sorted Cells
Sorted cells are typically used for molecular analysis or sample preparation, for example, cellular expansion for research or therapeutic purposes. In such cases, those cells need to be in a healthy metabolic state ideally retaining their complete array of functional capabilities. Current flow cytometric sorting has distinct challenges in that respect due to technical requirements such as high pressure, extended shear rate, severe decompression upon aerosolization, and impact trauma during cell collection. Often, these factors result in cell isolates with compromised function and/or viability.
Using Owl’s microchip-based technology, the pressure applied to cells is minimal and the trauma associated with droplet sorting is removed, resulting in high cell viability. In addition, a wide variety of cell types have been sorted using the Owl technology, all with a high retention of cell functionality. For example, antigen-primed T cells have been shown to retain their cancer-specific cytotoxic capabilities in chromium-release assays.
Microchip-Based Sorting
While the utility of microchip-based cell separation is being tested in many different applications, current studies show its utility in processing clinical samples for diagnostic and therapeutic applications. For these purposes it is best to process the samples with as little manipulation as possible.
Here, CD4+ cells were sorted from a diluted whole blood preparation by adding a fluorescent CD4 antibody marker to 8 mL PBS and 2 mL of whole blood. Results show that while the presorted fraction contained less than 0.01% CD4 positive cells, the sorted fraction contained more than 91% CD4 positive cells (Figures 3A and B). The effective purification was close to 10,000-fold in a single-step process and with a simple no-lyse, no-wash sample preparation.
Investigations have also been done to study the ability of antigen-specific T cells from a patient’s own blood to recognize tumor cells and trigger an immune system response. To explore the feasibility of this clinically important strategy, cells from a MART-1-specific T cell clone were spiked into a patient’s peripheral blood mononuclear cells (PBMCs), stained with a PE-conjugated tetramer loaded with a MART-1 peptide, and then sorted using the Owl’s microchip-based sorting technology.
Results show tetramer-positive cells were enriched from less than 1% to greater than 95% (Figures 3C and D). Importantly, sorted cells maintained their ability to kill MART-1+ tumor cells and to proliferate in vitro.
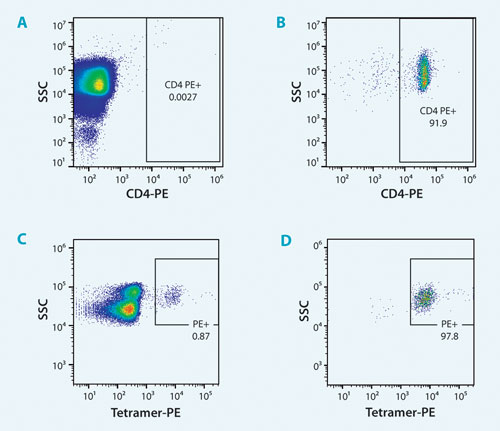
Figure 3. Sorting target cells from dilute whole blood or PBMCs: Pre-sort fraction of diluted whole blood with CD4+ cells (A) Post-sort fraction showing enrichment of CD-4 positive cells (B) Pre-sort fraction of the spiked MART-1-specific T-cell clone into PBMCs (C) Post-sort fraction showing enrichment of MART-1 positive antigen specific T cells (D).
Conclusions
Acceleration of research for effective cancer and cellular therapies has increased the need for cell-sorting technologies that are safe, efficient, and easy to use. Owl’s cartridge-based closed system has effectively demonstrated its capability to sort a wide variety of cells at high speeds without impacting cell viability. This platform offers the additional advantage of protecting sample integrity while permitting quick sample-to-sample changeover—a feature that avoids the inter-sample cleaning and validation required for traditional cell sorting.
That this technology does not utilize sheath fluids opens the future potential for sequential sorting of large numbers of samples within a short timeframe, making practical clinical applications a possibility. In addition, combining the underlying microchip technology with other cell isolation techniques such as magnetic beads has the potential to empower processing of samples greater than 1 billion cells.
Jim Linton, Ph.D. ([email protected]), is chief business officer at Owl biomedical, and Shane W. Oram, Ph.D. ([email protected]), is global marketing manager—cell analysis at Miltenyi Biotec.



