February 15, 2013 (Vol. 33, No. 4)
Richard Zimmerman
Tim Hamilton
Vinita Gupta, Ph.D.
Simultaneous Analysis of 18 Cancer Biomarkers Using Magnetic Beads
The Centers for Disease Control and Prevention estimates that one in every four deaths in the United States is due to cancer, yet approximately one-third of all cancer cases could be prevented if detected early enough. Thus, there is a growing need to develop new tools for the study of cancer biomarkers as indicators of disease mechanism, disease progression, and drug efficacy.
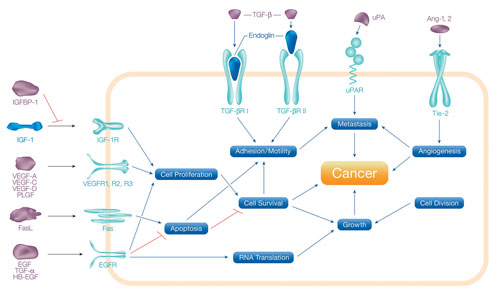
Of particular interest to the life science research community are multiplex assays capable of measuring soluble proteins involved in cancer-related processes such as angiogenesis, metastasis, cell proliferation, cell adhesion, apoptosis, and inflammation (Figure 1).
Using Luminex xMAP technology we developed Bio-Rad Laboratories’ Bio-Plex® Pro human cancer biomarker panel 2, which employs a magnetic bead-based workflow to quantify cancer biomarkers in diverse matrices including serum, plasma, cell culture supernatant, and many other sample types.
In combination with the Bio-Plex readers and the Bio-Plex Pro wash station, this multiplex panel makes it possible to quantify 18 analytes in a single well of a 96-well microplate in only 3 hr with a sample volume of just 12.5 μL. The 18 markers were selected for their direct relevance to tumor-associated angiogenesis and related processes.
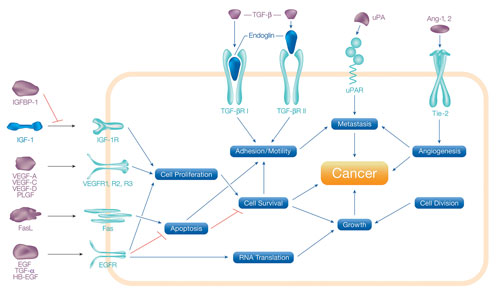
Figure 1. Biological processes involved in the development and progression of cancer
Method
This bead-based assay is performed in a 96-well microplate using principles similar to a sandwich-based capture immunoassay, as described in Bio-Rad bulletin 6156. The antibody-coupled capture beads were first incubated with multiplexed antigen standards or samples followed by a wash step and then incubation with biotinylated detection antibodies. After washing away the unbound biotinylated antibodies, the beads were incubated with a reporter streptavidin-phycoerythrin conjugate (SA-PE).
Following removal of excess SA-PE, the beads were passed through the Bio-Plex reader, which measures the fluorescence of each bead and the bound SA-PE. Assay incubations were performed at room temperature with a shaker speed of 850 +/- 50 RPM. Sample, detection antibody, and SA-PE incubation times were 60 min, 30 min, and 10 min, respectively. All washes were performed using a Bio-Plex Pro wash station. Data acquisition was conducted at the low photomultiplier tube (PMT) setting (LOW RP1 in the Bio-Plex Manager™ 6.0 software).
Assay Performance Characteristics
The assays were validated with biological samples and evaluated according to the following parameters: sensitivity, precision, accuracy, working range, cross-reactivity, and matrix effects. Assay sensitivity, defined as the limit of detection (LOD), was determined by adding two standard deviations to the mean median fluorescence intensity (MFI) of ten zero-standard replicates. With the exception of VEGF-D, all the analytes were detected at <5.7 pg/mL in both a serum-based standard diluent and RPMI cell culture medium. VEGF-D had an LOD of 11.5 pg/mL.
Assay reproducibility evaluates both the intra-assay and inter-assay coefficients of variation (reported as a percentage, %CV). The intra-assay (within-run) %CV assesses the variation among the replicates within the assay, whereas the inter-assay %CV measures the variability across three independent assays.
Intra-assay %CV values were calculated from the MFIs of all three replicates at each standard dilution point from representative assays in both serum-based standard diluent and RPMI matrices. All the analytes exhibited <7.4 %CV in both the serum and RPMI matrices. The inter-assay %CV for multiplex assays was determined from three independent plates using the observed concentrations of six-level spiked controls or the quality controls supplied in the kit. With spiked controls, analytes showed <9.6 %CV in both serum and RPMI. The quality controls exhibited <18.2 %CV in standard diluent.
Assay accuracy (also defined as recovery) was calculated as the percentage of the observed concentration value of an analyte relative to the expected value. This parameter was evaluated using standard concentration points and spiked controls in both multiplex and singleplex configurations and in both serum and RPMI. Overall, the standard recoveries were comparable in both matrices, with the majority of analytes showing recoveries of 80–120% within the assay working range.
Assay working range is defined as the range between the lower limit of quantification (LLOQ) and the upper limit of quantification (ULOQ) wherein an assay is both precise (an intra-assay %CV ≤10) and accurate (80–120% of the standard curve recovery). The ranges of these assays were determined for both serum and RPMI cell culture medium.
The Table lists the assay ranges for multiplex and singleplex assays in both serum and RPMI cell culture medium. The reproducibility of these ranges is dictated by the overall precision in preparing the assay reagents.
Assay specificity was examined by performing single-antigen and single-detection cross-reactivity studies. The single antigen study evaluated the specificity of a capture antibody by testing an individual antigen in the presence of multiplexed capture beads and the detection antibody. The single detection study evaluated the specificity of both the capture and detection antibodies by testing each individual detection antibody in the presence of multiplexed antigens and capture beads. The data analysis utilized weighting of the second-highest standard concentration point. The results showed that the degree of cross-reactivity across the entire panel was <5%.
Dilution linearity was assessed by spiking known quantities of recombinant antigens into human serum and plasma samples in a 1-in-3 serial dilution. For analytes typically present at high endogenous levels, serum or plasma (without spiking) was diluted with sample diluent in a 1-in-4 serial dilution followed by subsequent dilution in standard diluent to maintain a 25% serum content. The observed sample concentrations were plotted as a function of their expected concentrations within the assay working range to derive correlation coefficient (R2) values. All the analytes exhibited a correlation coefficient >0.9 in both singleplex and multiplex format.
Assay parallelism was investigated by comparing the slopes of spike concentration-response curves in human serum or plasma matrices with that of the standard curve using four-parameter logistic (4-PL) curve fitting. The percentage differences in slope value with respect to standard curve were less than 25% for all analytes in the panel.
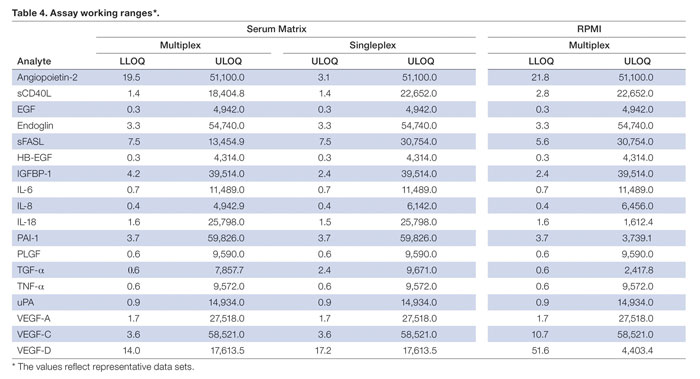
Assay working ranges (The values reflect representative datasets.)
Assay Validation with Biological Samples
The multiplex assays were further validated by conducting panel measurements in normal and disease serum and plasma samples. Figure 2 presents heat maps of the relative levels of biomarkers, indicating the P-values across the entire panel for breast cancer serum (n = 26 total) and colon cancer serum (n = 32 total); 15 of the 18 markers were elevated in colon cancer serum. The samples represent both treated and untreated patients and may explain the marginal separation between control and cancer for several markers (P-values >0.05). No P-values were obtained for TGF-a due to the low or undetectable levels in normal samples.
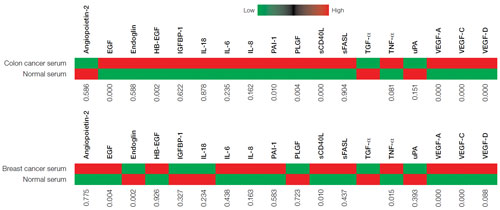
Figure 2. Heat maps for breast and colon cancer
Conclusions
The Bio-Plex Pro human cancer biomarker panel 2 is a magnetic bead-based multiplex assay panel designed to meet the needs of the most discerning academic and pharmaceutical researchers. The multiplex format provides a global view of angiogenesis and other important cancer-related processes in one simple assay, with an emphasis on data quality and reproducibility. The validation studies described here demonstrate the robustness of all 18 assays and further show that the simultaneous measurement of multiple markers greatly reduces the time, cost, and sample volume required compared with traditional systems such as enzyme-linked immunosorbent assay (ELISA).
Richard Zimmerman (Richard_Zimmerman @bio-rad.com) and Tim Hamilton (Tim_ [email protected]) are chemists, Vinita Gupta, Ph.D., is a staff scientist. All three authors work in the protein function division of Bio-Rad Laboratories.