September 15, 2010 (Vol. 30, No. 16)
Kate Marusina Ph.D.
Solution Providers Address Need for Increased Speed, Reduced Cost, and Easier Patient Access
At its inception, the term “molecular diagnostics” was narrowly applied to DNA- and RNA-based test and methods. The meaning of molecular diagnostics, however, has since expanded to include other molecules such as proteins and metabolites.
Rapid incorporation of scientific discoveries into diagnostics kits has led to a proliferation of complex analyses, often with multiple different analytes. Regardless of the term, an impressive amount of research is now focused on developing diagnostic applications that can help medical practitioners achieve faster and more accurate diagnosis, reliably assess disease prognosis, and monitor treatment.
Multiple opposing forces are shaping technology trends in molecular diagnostics. Significant advances in sample preparation and signal amplification have made diagnostic assays based on a single-copy or low concentration targets possible. As the technical complexity of assays increases, the diagnostic frontier is shifting from specialized labs and company-based CLIA labs to physician offices and even further into the field, to those dealing with major public health threats, military operations, or to patients’ homes.
Users are demanding fully integrated assay platforms that require minimal training to use and provide easy interpretation of results. Field applications demand low-cost solutions, which are contrary to the increasing costs of obtaining sufficient clinical evidence and traditionally low rates of reimbursement. The high clinical value offered by modern diagnostic systems underscores the long-overdue need for the overhaul of the reimbursement system.
In the interim, the quest for cost savings is further pushing the limits of technology to miniaturize the reactions, to increase multiplexing, and to improve signal amplification. The companies described in this article, all of whom will present at Select Biosciences’ upcoming “Molecular Diagnostics World Congress,” are capitalizing on these trends by offering unique solutions to the challenges of clinical in vitro diagnostics.
Polymer Laminate Technology
Microfluidics is a framework of micro- or nano-size channels enabling fluid flow for separation and analysis of macromolecules. The test cartridges within the device enable various functions such as automatic sampling, sample transport, any necessary chemical reactions, and detection on a single, miniaturized platform.
The advantages of microfluidics clearly support the needs of modern diagnostics by offering faster separations, shorter transport time, lower sample and reagent consumption, and the possibility of multicomponent testing under the same conditions.
To truly serve diagnostic needs, however, microfluidic platforms need to deliver fully integrated micrometer-scale systems, while maintaining biological compatibility, biomolecule stability, and extremely sensitive detection.
Material properties impact not just mechanical performance but also fluid behavior. The surface energy and geometry of the channel features also influence the fluid flow. Therefore, the choice of material and design of the cartridge and device are of paramount importance. But the huge amount of materials available for microstructure fabrication makes the selection difficult, especially for those not intimately familiar with polymer chemistry.
“Innovations are required from microfluidics manufacturers to reduce the price per cartridge from a typical $20 to a more sustainable price of $5 at launch, and further to $1.00 or less at high volumes,” comments Leanne Levine, founder of ALine.
“Such innovations come not only from novel materials, but also from integrated design-build-supply capabilities. This has necessitated a paradigm shift from silos of expertise in microfabrication to a wholly integrated manufacturing infrastructure.”
ALine’s mission is to innovate microfluidic platform enginering by vertically integrating a full suite of necessary functions, including novel material technology (polymer laminate; PLT), rapid prototyping, manufacturing, and the supply chain network.
The PLT process begins with a 2-D CAD design “split” into necessary layers, 12.5 micron to 3 mm thick. The layers are designed to incorporate necessary microfluidics features such as channels, valves, pumps, and filters. Different stock polymer materials can be used for each layer. The layers are cut with CO2 gas lasers and stacked and bonded using pressure-sensitive adhesives.
ALine’s fabrication approach simplifies connection to the valves, membranes, sensors, and other interfaces. “The eSENSOR® Genotyping cartridge for warfarin sensitivity testing developed by ALine and GenMark Diagnostics combines polymer laminate layers containing complex fluid channels with the injection molded part for sample loading and the electrical sensor circuit,” Dr. Levine continues.
“This product required numerous design iterations to empirically optimize pumping functions and material selection. But now we have the capacity to produce 25,000 parts per month in our current facility.”
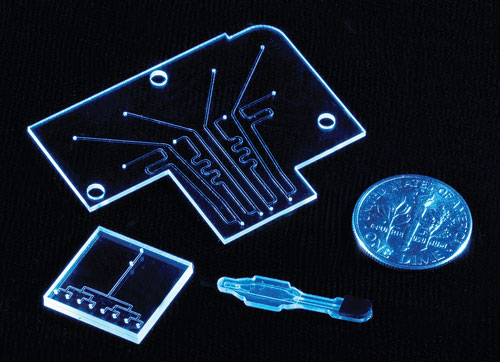
According to ALine, its polymer laminate technology platform enables rapid prototyping and volume manufacture of biomicrofluidic devices.
GC-Rich Sequences
TriLink BioTechnologies has long supplied reagents to the molecular diagnostics field and “is now uniquely poised to play a more direct role,” says Natasha Paul, scientific investigator.
“Our CleanAmp™ hot-start PCR technology can support applications in the diagnostics field that require high specificity of target amplification.” TriLink’s CleanAmp dNTP technology is based on the use of a 3´-thermolabile dNTP protecting group that prevents primer extension at low temperatures. The protecting group is removed during the hot-start activation step of PCR, increasing the specificity of amplification of the intended target.
As molecular diagnostics trend toward advanced amplification strategies, new targets are often located in highly GC-rich regions, including promoter and enhancer sequences known to play a pivotal role in gene regulation.
Assays targeting these regions are often plagued by low-amplification specificity. Substitution of dGTP with 7-deaza-dGTP has improved specificity by disrupting complex secondary structure formation within these sequences.
TriLink BioTechnologies has made this valuable tool into a hot-start solution called CleanAmp 7-deaza-dGTP. Reactions with CleanAmp 7-deaza-dGTP significantly improved amplification specificity for targets containing as much as 84% GC content, Paul reports. “We are encouraged by these results and are exploring development of reagents for clinically relevant targets.”
Over the past few years, point-of-care (POC) applications have became a significant driving force in the diagnostics field. In part, this is being driven by aging patients and a growing need to perform routine diagnostic analysis at home or at specialized-care facilities.
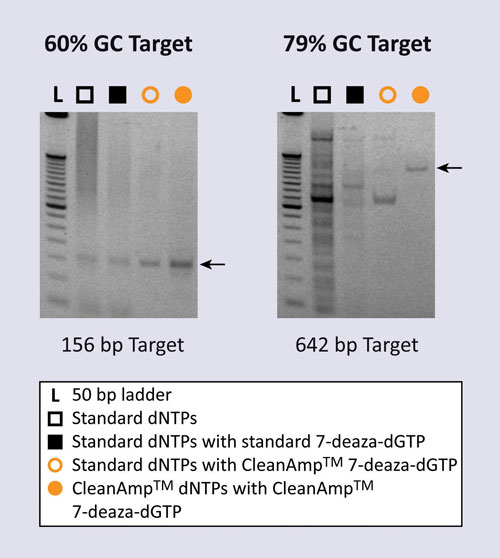
TriLink BioTechnologies’ CleanAmp™ dNTPs are part of the firm’s line of PCR-enhancing products. CleanAmp supports applications in the diagnostics field that require high specificity of target amplification.
Point-of-Care Device
“A host of other human health, animal health, and environmental needs could be addressed by point-of-care testing,” notes Keith Page, CEO of Argento Diagnostics. “However, achieving true portability while maintaining quality on par with a large clinical laboratory remains a challenge. A diagnostic system in field situations must accept a variety of common sample sources, often with poor quality.”
Argento’s platform technology can be compacted to the size of a typical smart phone. The touch-screen interface requires minimal technical savvy, and the data can be transmitted via Internet. Miniaturization of Argento’s technology is made possible by a novel detection method that amplifies the signal 106 times.
According to the company, its electrochemical immunoassay system is capable of detecting 30 viral particles in 1 mL of nasal lavage. Argento utilizes a familiar immunoassay with the analyte captured on antibody-coated magnetic beads.
The complexes are detected by silver nanoparticles composed of 106 silver ions coated with a specific binding molecule. The unique attribute of this technology comes from the detection of silver ions by anodic stripping voltammetry. This method involves coating the silver particles with ammonium isothiocyanite that attributes a negative charge to the particles.
The coated particles are drawn to the positively charged electrodes on the assay chips, where the particles are dispersed into silver ions. The number of silver ions is directly proportional to the number of complexes.
Argento’s technology eliminates the need for harsh oxidative chemicals used to generate silver ions. The system can utilize copper, gold, and silver detection systems in a multiplex reaction with a common sample entry point. The time for analysis depends on the viscosity of the sample. An assay based on saliva takes about three to five minutes, Page says.
“The use of proteins for diagnostic tests is typically limited by the detection methods, which are only sensitive enough when the protein concentrations have already reached critical thresholds and disease has progressed. Our current detection capabilities are on par with clinical labs, and the detection could be improved further depending on a clinical need.”

Argento Diagnostics, a spinout from National Physical Laboratory, has launched an analysis technology that reportedly enables multiple quantitative measurements to be made from a single sample (e.g., blood, urine, or saliva) in a fully portable handheld device.
Antibiotic Susceptibility
The gold standard for pathogen detection is a phenotypic approach whereby the samples are cultured in growth media with or without different antibiotics. Although specific, the test takes 48–96 hours to complete, potentially missing the narrow treatment window. According to Vincent Gau, Ph.D., co-founder, CEO, president, and CTO of Genefluidics, his company is approaching clinical validation of a biosensor system that would enable it to shorten diagnosis time to one to two hours.
Genefluidics’ systems use crude, unseparated samples that reduce the processing time and increase specificity. However, detection of unamplified targets present at low concentrations requires highly sensitive detection methods.
“Optical detection methods measure optical density, but crude samples are often cloudy. We utilize all commonly used ELISA reagents not in optical but in electrochemical detection,” explains Dr. Gau.
A detector probe (protein or nucleic acid) is conjugated with horseradish peroxidase (HRP), which is oxidized by hydrogen peroxide and reacts with one molecule of the TMB substrate, a commonly used ELISA substrate. A working electrode supplies an electron to reduce one TMB molecule, and the cycle starts over again.
HRP cycles through 10,000 Redox steps per second generating strong electrical signals in a relatively short time span. Validation with urine samples of patients with suspected urinary tract infections confirmed 100% accuracy of the HRP electrochemical assay compared to clinical laboratory results.
“The sensor chips are designed to detect RNA and proteins simultaneously, as well as internal controls,” adds Dr. Gau. “Internal controls are critical to monitor reagent quality and time sequence of reagent delivery. Our systems continuously measure electrical impedance for internal quality control.”
Genefluidics has a POC system with 6 sensors per cartridge and a lab system with 16 sensors per cartridge. According to the company, the genetic assay is able to detect femtomolar quantities of nucleic acid without target amplification.
Gen-Probe was the first company to have an FDA-cleared nucleic acid test (NAT) in the ‘80s, says Michael Watts, vp of investor relations. “We are now the world’s largest stand-alone molecular diagnostics company. The cornerstone of our success is integration of several essential technologies with fully automated instrumentation.”
Gen-Probe’s transcription-mediated amplification (TMA) technology involves multiple rounds of synthesis of RNA and DNA copies of the target. Each of the newly synthesized molecules serves as a template for a new round of replication, leading to exponential expansion of the amplicon. This expansion can result in the production of billions of amplicons in less than one hour.
“Our technology is well positioned in areas where specificity and sensitivity requirements are paramount,” continues Watts. “For example, our blood screening test for West Nile virus has an analytical sensitivity of 9.8 RNA copies per mL of blood, with 99.91 percent specificity.”
Watts also reports that Gen-Probe’s high-throughput system, Tigris, is able to process about 500 patient samples within one eight-hour shift.
The company’s new Panther instrument preserves the automation of Tigris and is geared to lower volume hospital labs. Panther, which is expected to be launched in Europe around year-end, reportedly processes 275 samples in 8 hours, with up to 120 tubes being loaded at the same time.
Panther is able to run both qualitative tests (such as Chlamydia and gonorrhea assays) and quantitative tests.

Gen-Probe’s Panther is physically smaller than the company’s Tigris system and is geared to lower volume hospital labs. It incorporates several features such as random access sample loading that make it more user-friendly for lab technicians. In addition, it has the flexibility to run real-time assays.







