August 1, 2006 (Vol. 26, No. 14)
Ceramic Hydroxyapatite Gains Following as Critical Component in Purification Process
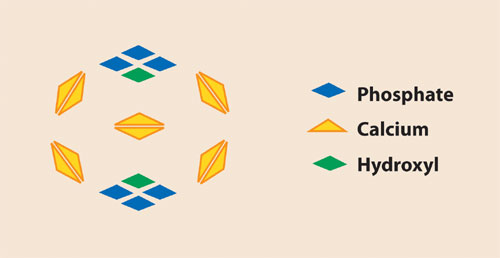
Hydroxyapatite (HAP) is a crystalline material consisting of Ca2+ and PO43- groups in the lattice (Figure 1). Unlike other matrices used for liquid chromatography that are composed of reactive ligands affixed to the matrix, HAP is both the ligand and the matrix.
HAP was originally made in the 1950s by the Tiselius method. Adjustments made thereafter often resulted in excess phosphate present in the structure. This causes the formation of unstable, rectangular, plate-shaped crystals with poor flow, pressure, and stability characteristics.
Recently developed synthesis methods yield hexagonal cross-section, columnar crystals with the ideal Ca:P ratio of 1.67:1. They can be agglomerated to form particles and sintered at high temperatures to fuse the particles into a stable, porous, ceramic mass. This yields ceramic hydroxyapatite (CHT) media that possess several important properties, such as macroporous structure that provides a large surface area, limited mass-transfer resistance, high mechanical strength, and base resistance.
Such important attributes make CHT commercially attractive and competitive with other popular chromatography resins for laboratory and process applications. Furthermore, the main constituents of CHT are virtually the same as those of human bone, thus making leachables from the resin irrelevant.

Fig.1: The chemical structure of CHT consists of five positively charged calcium pairs, two phosphates triplets that each have six negatively charged oxygen atoms, and two hydroxyl residues.
Advantages of CHT
The well-known affinity of DNA for HAP has made HAP an effective tool for DNA removal and purification. DNA binds HAP by the metal interaction of DNA phosphates with crystal calcium. Electrostatic repulsion between crystal calcium and DNA phosphates reduces the net binding energy. The latter effect is damped with the addition of NaCl, causing DNA retention to increase.
Like DNA, endotoxins also bind to CHT primarily because of the phosphate moiety present in lipopolysaccharides. Lipopolysaccharide is a main component of the outer cell membranes of gram-negative bacteria, such as E. coli, Salmonella typhosa, Salmonella typhimurium, and Bordetella pertussis. If not removed from solutions intended for intravenous applications, lipopolysaccharides can cause serious physiopathological effects in humans.
Besides its interaction with DNA and endotoxins, CHT stands out due to its ability to surmount two additional key challenges experienced in the purification of Mabs: removal of leached protein A and removal of product aggregates.
Removal of these is essential because of possible toxicity in the case of protein A and increased occurrence of neutralizing antibodies in the case of product aggregates. Protein A is affinity-complexed to the Mab, and the complex is eluted later than is the uncomplexed antibody. Product aggregates are removed due to their enhanced retention on CHT. With proper management of pH and phosphate conditions, monomeric IgG can be successfully separated from the aggregates.
With regard to how IgG binds to CHT, previous work demonstrated retention on HAP by a combination of two major mechanisms: phosphoryl cation exchange, which involves the interaction of positively charged amino groups on proteins with negatively charged phosphate groups on the matrix, and calcium–metal affinity, which involves interaction between the protein carboxyl groups and the positively charged calcium groups on the matrix. Although the initial attraction of the latter mechanism is electrostatic, the eventual coordination bond between calcium and protein carboxyl groups is much stronger.
Partner for Protein A
Application data have now established CHT as the ideal partner for protein A in the purification of Mabs. The method that has been developed can be adapted easily to many classes of antibodies, including mouse Mabs, human IgG1s, and IgG4s, with a wide range of isoelectric points, mouse-human IgG chimeras, and IgMs. CHT permits simultaneous removal of aggregates, leached protein A, DNA, and endotoxins (Figure 2).
In addition, considerable clearance of host cell proteins has been established, although the data are not as clear-cut, because host cell proteins comprise an assortment of both basic and acidic proteins. Depending upon the binding and elution conditions, clearance of host cell proteins can occur in cooperation with the clearance of other contaminants.

Fig.2: CHT fractionation of contaminants is shown; the light blue zone contains monomeric IgG.
Viral Clearance
Results of a recent study sponsored by Bio-Rad (www.bio-rad.com) demonstrated the effectiveness of CHT for viral clearance. More than 1,000-fold clearance of xenotropic murine leukemia virus (X-MuLV) and 100-fold clearance of minute virus of mice (MVM) were realized.
The selection of viruses is in compliance with the recommendations provided by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, as well as in the Committee for Proprietary Medicinal Products documents on virus validation studies. The clearance data is shown in the Table.
These results were achieved with protein-A-purified human IgG1 Mab loaded on a column of CHT type I, 40 microns. The sample was mixed with virus, applied in 10-mM sodium phosphate, pH 6.5, and then eluted with a 10-column-volumes linear gradient to 2-M NaCl. The column was then cleaned with 10 column volumes of 0.5-M NaPO4, pH 6.5. These conditions are consistent with the range recommended for removal of aggregates, leached protein A, DNA, and endotoxins.
The X-MuLV (an enveloped retrovirus) data are most relevant because of the potential for endogenous retroviruses to be present in the CHO cell lines that are used for antibody production and for other mammalian protein production. Thus, these data are of universal interest to purification process developers.
MVM is a nonenveloped parvovirus with high resistance to physicochemical inactivation. It is not associated with mammalian production systems but is used to establish the ability of the process step to remove a virus rather than to achieve a specific removal goal.
Elution of the majority of both viruses in the 0.5-M phosphate cleaning step is very positive because it indicates that a similar degree of virus clearance can be expected from different Mabs, as long as they are eluted within the NaCl gradient. Every Mab examined up to this date can be eluted with a NaCl platform, and the process-development strategy suggested ensures that other Mabs will follow this pattern.
These data also indicated that equivalent viral clearance can be obtained when CHT is conducted in a flow-through mode, as long as the phosphate concentration is kept low and selectivity is controlled with NaCl. The data presented demonstrate the feasibility of using CHT for viral clearance, but it will remain the process-developer’s responsibility to validate clearance for specific products and conditions.
Clearance obtained with the CHT/NaCl gradient system is as good as or better than that which is achieved with other chromatography techniques. In the context of its ability to simultaneously remove aggregates, leached protein A, DNA, endotoxins, and host cell proteins in combination with virus removal, however, CHT stands alone.
CHT’s resolution property makes it a powerful tool for process developers. The ability of CHT to purify a variety of proteins, including Mabs that are leading licensed products or therapeutic candidates in many drug companies, highlights its versatility. It is anticipated that CHT will gain even more favor as a component in purification processes.