June 1, 2009 (Vol. 29, No. 11)
Genmab Relies on IDBS’ ActivityBase XE for Analyzing Results from Its Discovery Activities
Therapeutic monoclonal antibodies are the fastest growing segment within the pharmaceutical market. Among their important attributes is their high specificity. The ability to target specific antigens (expressed on cells, tissues, and organs) involved in the pathology of disease minimizes side effects and contributes significantly to the popularity and success of monoclonal antibodies in clinical applications.
Unlike several conventional (small molecule) therapies that offer only short-term symptomatic relief and can potentially cause serious side effects, it is believed that monoclonal antibodies provide more effective treatment with greater efficacy and tolerability.
Genmab creates and develops fully human monoclonal antibodies using UltiMab® technology licensed from Medarex. Therapeutic antibodies produced using this technology consist of 100% human proteins. High-quality, high-affinity human antibodies can reportedly be rapidly generated in a matter of months and can be selected to bind to naturally occurring human target proteins, tumor cells, or infectious agents. The UltiMab technology eliminates the need for humanization or complicated genetic engineering, which can be time consuming and expensive, according to the company.
ActivityBase XE
Genmab initially purchased IDBS’ ActivityBase® Xtended Edition (ActivityBase XE) to handle the design, capture, storage, and retrieval of its plate-based screening data (Figure 1). Genmab’s use of ActivityBase XE was later extended to its hybridoma discovery department, which sought a flexible data-management solution to handle functional screening data of human monoclonal antibody therapies.
Using the screening solution to capture, validate, and visualize antibody screening data, Genmab’s scientists employ ActivityBase XE to perform plate-based serum, primary, and secondary/functional screening assays, with a specific plate layout applied to each assay type.
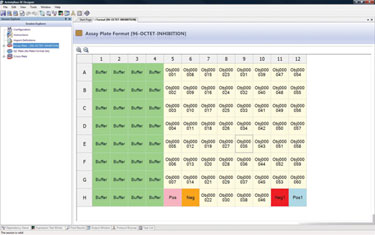
Figure 1. Genmab’s plate format
For all the screens, the data analysis and visualizations performed are used to determine the quality of the screen and to select hits for the next screen. At each phase, the information generated is used to determine which antibodies are to be moved to preclinical assays (Figure 2).
The serum-screening process begins with the extraction of serum samples before immunization. This first serum sample obtained is called pre-tap, which is used in every titer screening as a reference sample for that particular subject. After immunizations start, when subjects start to produce antibodies, scientists run the serum-screening assay every two weeks to collect serum samples referred to as tap.
The first step in selecting a hybridoma cell that produces antibodies with the desired characteristics involves plating out cells in different wells. Employing a highly automated and efficient process, Genmab uses Caliper Life Sciences’ Sciclone liquid-handling workstation to pipette the diluted samples into 384-well assay plates, which are filled up with a cell line expressing the target of interest and a control mock cell line.
These plates are then moved to Cello™ (The Automation Partnership), a high-throughput fully integrated imaging and culture system that can handle large hybridoma libraries. In a highly controlled manner, cells grow in wells and produce significant amounts of antibodies, which are then ready to be tested for their specificity and functional characteristics.
To determine potential antibody hits to progress to a primary screen, graphical information is compiled that visualizes serial dilutions of the tap and pre-tap on the target and the mock cell line, per individual subject and for the entire group. Trend analysis of the positive and negative controls within one plate and on all plates in one run is also performed.
For the immunization protocols performed subsequently to the serum screening, ActivityBase XE is used to collate similar graphical information, plus trend analysis of positive and negative controls over time. This analysis helps scientists to detect the subjects that are producing the antibodies with the desired characteristics.
When subjects are identified as producing potential hits, lymph and spleen cells are fused in a fusion protocol. Fused cells are placed in fusion plates in 96-well format and moved to the automated culture robot.
After fusion, several compound plates (harvest) are produced in the automated culture robot, which also outputs culture supernatants. Together with the output plates, a worksheet (fusion screening sheet) that contains all calculated assay data from the primary screening is produced, which is used as a basis for decisions for candidate selection in the next phase.
Scientists then perform primary screens on the supernatants, and the captured data is analyzed, which also allows further trend analyses on the positive and negative controls within one plate and on all plates in one run. Combined with the trend analyses of the on-plate effect, this information is used to help identify patterns of behavior within and across plates and to compare these trends in order to detect system errors.
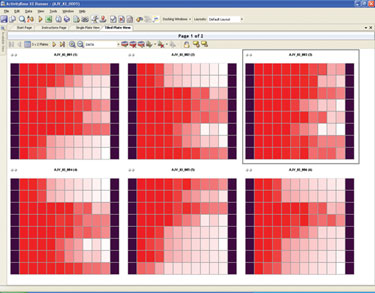
Figure 2. ActivityBase XE tiled dose response
The hits from the primary screen that display the best therapeutic characteristics are isolated and purified before going through a secondary/functional screen. The assay results are used to determine the IC50/EC50 values for the samples, which gives scientists an indication of how effectively an antibody binds to a target (Figure 3).
Scientists visualize the secondary/functional screening data to depict a graph per sample showing serial dilutions of the samples, the target expressing cell line and the mock cell line, plus a graph with all the samples in the run and the corresponding IC50/EC50 values for both the cell lines.
Trend analysis of the positive and negative controls within and across plates in the run is again carried out. This data provides scientists with an indication of an antibody’s ability to bind to its target, allowing identification of hits with the best binding properties.
In addition to supporting traditional high-throughput and high-content screening, ActivityBase XE can accommodate a wide range of screening methodologies, offering Genmab the flexibility it needs to meet its hybridoma discovery data requirements.
ActivityBase XE ensures that data is transparent across screens and can be accessed and retrieved at any stage for reference or further analysis. All screening data is captured and stored in one accessible location, which provides context around the hybridoma discovery department’s screening results, leading to better understanding and enhanced decision making.
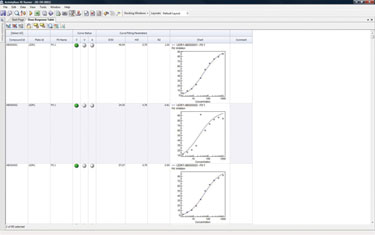
Figure 3. Assay results were used to determine the IC50/EC50 values for the samples.
Andy Vines ([email protected]) is scientific software manager at IDBS. Web: www.idbs.com.



