June 15, 2013 (Vol. 33, No. 12)
Biotechnology primarily focuses on creating novel biologic therapeutics, and there’s no doubt that bioresearch has led to a number of life-improving and life-saving drugs. Indeed, last fall the European Medicines Agency approved the first gene therapy in the Western World—Glybera—for the treatment of lipoprotein lipase deficiency.
However, traditional small molecule drugs remain a big part of the therapeutic landscape. For example, Global Industry Analysts predicts that the world market for small molecule kinase inhibitor drugs will reach $16.2 billion in two years, while Visiongain estimates that small molecule targeted cancer therapy revenues will total $32.7 billion worldwide in three years, up from $21.7 billion in 2011. Hardly chump change.
Thus, research on small molecule drugs continues at a rapid pace, as evidenced at CHI’s recent drug chemistry conference in San Diego. Several scientists presented their latest findings on biological and chemical investigations and characterization of specific small molecules as potential inhibitors, antagonists, or markers of target proteins.
According to Jan Hoflack, Ph.D., CSO and head of drug discovery at Oncodesign Biotechnology, his firm’s Nanocyclix platform aims to develop small macrocyclic molecules with potential drug effects against kinases.
“This class of proteins, based on their inherent capacity to send signals from the extracellular environment to particular proteins within the cell, thereby regulating physiological responses, can be used as targets for therapeutics. Our chemistry-driven approach to target selection, coupled with chemogenomics techniques, has allowed us to screen approximately 5,000 compounds that present different scaffolds and linkers, which we know are key to increasing the efficiency of inhibitory effects,” Dr. Hoflack explained.
“We identify compounds of interest based on selectivity, potency, and novelty of the kinase targets they interact with. This chemistry-driven approach to identify macrocylic kinase inhibitors moves away from the conventional approach of performing high-throughput target-based assays on pre-defined kinases by focusing on the intrinsic chemical profiles of these small compounds, opening up a target space that has been unexplored in kinases.”
Dr. Hoflack also said that small macrocyles offer unique features that increase their potential as therapeutics.
“These macrocycles possess high selectivity, which is attributable to three-dimensional shape complementarity and reduced conformational freedom, thus allowing them to fit as a hand in a glove into the ATP-binding site of kinases. This unique selectivity thus offers potential in a wide range of therapeutic applications, not only cancer, but also auto-inflammatory and neurodegenerative conditions such as irritable bowel disease, Crohn’s disease, rheumatoid arthritis, Parkinson disease, and Alzheimer’s,” Dr. Hoflack said.
He noted that more than half of the human kinome remains unexplored by current inhibitor approaches. “Current research efforts on kinases cover only a small proportion of the kinome and although these have generated inhibitors with strong biological activities and major benefits for patients, their application has mainly been in oncology.
Oncodesign has identified specific inhibitors that may present increased effectiveness and safety, and thus may be considered as next-generation inhibitors in cancer but also in other therapeutic areas. The novelty of our chemistry-fueled approach allows us to position it in areas with major unmet need by identifying clinically relevant molecules for patients that currently have no treatment options,” Dr. Hoflack said.
Working in collaboration with pharma teams such as Ipsen and Sanofi, Oncodesign is currently developing inhibitory compounds of the leucine-rich repeat kinase 2 (LRRK2), receptor interacting protein 2 (RIP2), and salt inducible kinase 2 (SIK2).
“We currently hold about 20 patents for novel kinase inhibitors that possess the unique properties of potency and selectivity while maintaining very small molecular weights, giving them drug-like properties that are difficult to achieve with nonmacrocyclic compounds,” Dr. Hoflack said.
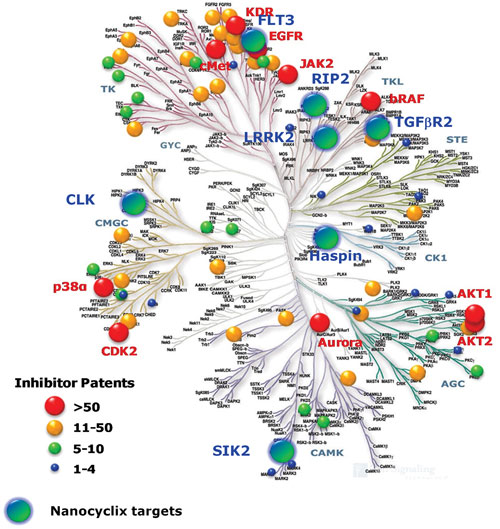
Oncodesign Biotechnology uses its Nanocyclix® platform to generate compounds with selectivity profiles that do not exist elsewhere, it says.
Protein-Protein Interactions
The applications of macrocycles have also been investigated at Ensemble Therapeutics, a firm that is treating these as ideal compounds to address extended-binding site targets found in protein-protein interactions.
According to Nick Terrett, Ph.D., CSO, “There are a compelling number of drug targets where macrocycles have the potential to bind with good affinity. These compounds, which generally have a molecular weight range of 500 to 1,000 Daltons, can disrupt tough protein-protein interactions and thus present a novel and high potency approach to new drugs for significant medical conditions.”
“These macrocycles are typically smaller than biologic drugs such as antibodies, yet larger than conventional small molecules, but can provide us with specific positive attributes from each category—the potency and selectivity of biologics but in an orally bioavailable small molecule package.”
For example, scientists at Ensemble have successfully pursued research investigations to identify compounds that target interleukin-17A (IL-17A), as well as other proteins, that inhibit inflammatory responses associated with psoriasis and other chronic disorders.
“The use of macrocycles as potential drug therapies confers two major advantages. First, the oral bioavailability of these compounds was significantly increased as demonstrated in our studies using animal models. Our vision is to design a drug that is not only potent and selective, but also patient-friendly, such as a pill that could be self-administered and is obviously less invasive than an injectable,” Dr. Terrett explained. “Second, these macrocycles can readily penetrate cell membranes and interact with intracellular protein-protein interactions, as well as extracellular proteins, thereby greatly broadening the utility of these novel drugs.”
Dr. Terrett and his colleagues have employed DNA-encoded chemistry—a technology that involves the use of specific DNA sequences to make thousands of synthetic macrocyclic compounds in a single reaction sequence, which are then screened across various protein targets for their affinity and selectivity—to examine millions of macrocycles. Then, using PCR, the team was able to identify which macrocycles had bound to the target IL-17A protein.
“As soon as the binding affinity to the target protein has been determined, these potential macrocyclic compounds are subjected to additional biochemical, biophysical, and in vivo assays that further provide us information on their profile and drug-like properties. In particular, we pursue a rigorous medicinal chemical program to fine tune the macrocycles to optimize their solubility, permeability, and metabolic stability,” he noted.
“The generation and screening of our DNA-encoded collection of macrocycles can be considered as a game changer in the field because for the first time it allows us to identify orally bioavailable compounds that address tough protein-protein interaction targets across a range of important untreated medical conditions.”

Ensemble claims to be the first company to successfully and reliably exploit synthetic macrocycle drugs. Its Ensemblins™ are a new class of drugs in the emerging therapeutic space between small molecules and biologics.
Insights from Parasites
In the field of chromatin biology, small molecules have also played an integral part in assessing a cell’s activities.
Cheryl Arrowsmith, Ph.D., professor of medical biophysics and Canada Research Chair in Structural Genomics, University of Toronto, and members of her lab partake in the Structural Genomics Consortium (SGC), which focuses on large-scale cloning and characterization of human proteins and proteins from human parasites.
“We are trying to fill in the information that could not be provided by genomics analysis and by using a multidisciplinary approach, we have processed a large number of small molecules, as well as determined the activity of each compound in a biological context,” Dr. Arrowsmith said.
Her group has employed various techniques in structural biology to determine the 3D structures of proteins, as well as other screening and biophysical methods such as enzyme assays, fluorescence polarization, isothermal titration calorimetry, NMR spectroscopy, and thermal stability, to monitor interactions between proteins, peptides, and small molecules.
“Our current focus is on chromatin biology, attempting to uncover epigenetic mechanisms that influence basic biological phenomena that may be relevant to cancer therapy, inflammation, and neurobiology. In collaboration with pharmaceutical companies, we are developing unique small molecules—chemical probes—that selectively and potently inhibit specific chromatin regulatory proteins,” she explained. “A unique aspect of this project is that we, and our pharma and academic partners, make these chemical probes freely available for other scientists and researchers to study and better understand the relationship between biology and disease.”
In a recent publication in Nature Chemical Biology, Dr. Arrowsmith and her collaborators at the University of North Carolina described UNC1215, which is a potent, selective, cell-active small molecule that disrupts protein-protein interactions, a type of protein activity that has traditionally been considered difficult to drug.
“This chemical probe binds within a conserved protein pocket that normally binds methylated lysine groups (a common epigenetic chromatin posttranslational modification) resulting in the regulation of a specific protein (L3MBTL3) associated with brain tumors. UNC1215 thus prevents the interaction of methyllysine-modified proteins, such as histones, that regulate gene expression,” she said.
The SGC has also participated in other research studies involving the characterization of small molecules such as JQ-1, an antagonist of BET bromodomains, protein interaction modules that recognize acetylated lysine on histones, and important new drug targets in cancer and inflammation.
Kinase inhibitors have also seen extensive efforts in biological and chemical characterization. While this field has exploded over the past decade, many kinase targets remain unexplored in terms of inhibitor discovery. One example is the cytoplasmic tyrosine kinase encoded by the c-Fes proto-oncogene, which is a research focus of Tom Smithgall, Ph.D., professor of biochemistry and chairman, microbiology and molecular genetics, University of Pittsburgh School of Medicine.
“Together with Src and Abl, Fes was one of the first tyrosine kinases discovered many years ago. However, no inhibitors had been reported for this kinase despite its association with acute myeloid leukemia, multiple myeloma, and other forms of cancer,” Dr. Smithgall explained.
With his departmental colleague Sabine Hellwig, Ph.D., and collaborators Nathanael Gray, Ph.D., at Harvard Medical School, and SGC member Stefan Knapp, Ph.D., from the University of Oxford, Dr. Smithgall screened a small, kinase-based library of approximately 600 chemical compounds for inhibitory activity against the c-Fes kinase and identified eight classes of inhibitors with significant biological actions.
“From only 600 compounds, we found 20 to 30 compounds that showed potent activity against c-Fes in vitro and eventually ended up with about five compounds that helped us answer new biological questions about Fes,” Dr. Hellwig said. “Unexpectedly, we found a role for Fes activity in osteoclast differentiation from macrophages, suggesting possible roles for Fes in the osteolytic bone disease associated with multiple myeloma.”
She added that the team is currently using these inhibitors to look into the role of c-Fes in multiple myeloma and other blood cancers.
“It is possible that by utilizing this handful of small molecules against c-Fes, we might find additional roles and activities for c-Fes,” Dr. Hellwig said. She and Dr. Smithgall are now working with their collaborators to design second-generation compounds with even greater selectivity and potency against c-Fes.
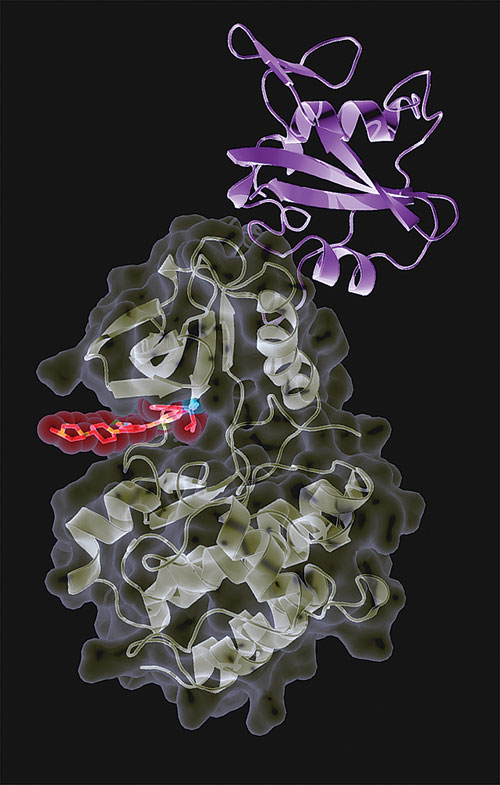
University of PIttsburgh researchers have been looking for inhibitors against the cytoplasmic tyrosine kinase encoded by the c-Fes proto-oncogene.



