March 15, 2006 (Vol. 26, No. 6)
The recognition of chirality in bioactive molecules is as old as modern organic chemistry itself. Pioneering work by Jean-Baptiste Biot, Louis Pasteur, Emil Fischer, and J. vant Hoff established chirality as an intrinsic property of molecules.
Despite the tragic experiences with racemic thalidomide in the 1950s, chirality did not play a significant role in pharmaceutical development until mid-1990s. At the time, large-scale asymmetric synthesis was impractical, so development of single-isomer drugs had to rely on inefficient chiral resolution. More recently, even with the emergence of new asymmetric synthesis methodologies, chiral active pharmaceutical ingredient (API) manufacture continues to be technically challenging. For a number of reasons, pharmaceutical manufacturers have been slow to adopt chiral technologies at the manufacturing scale.
Clearly, to be economically and chemically appropriate, introduction of chirality must occur in the context of the overall synthesis, production process, specific unit operations, and the manufacturers processing capability. More often than not, process-development groups at pharmaceutical companies face serious challenges when introducing chirality into complex pharmaceutical molecules.
The need for integrating chiral expertise with synthesis and process knowledge has never been greater. Approximately 30% of marketed brand drugs, half of all pipeline molecules, and three-fourths of new chemical entities contain chiral centers. Between 6070% of chiral pipeline molecules are fully synthetic. The rest semi-synthetic.
Challenges of Implementation
Chiral technology often originates in academic labs or small, resource-limited companies. We have observed many instances where an asymmetric reaction is strikingly effective in the laboratory, at small scale, but cannot easily be scaled to a manufacturing plant. The same shortcoming holds for chiral building blocks and intermediates, which often require a complete re-design of the process at significant cost in time and resources.
Chiral specialty companies must therefore recognize, before in-licensing chiral technology, the difference between a reaction and a process. Chiral Quest (www.chiralquest.com) kept this distinction in sight when, in 2000, it decided to commercialize several asymmetric hydrogenation catalysts from Xumu Zhang, Ph.D., a chiral chemistry specialist at The Pennsylvania State University.
By early 2005, Chiral Quest had developed synthesis routes for nine of Dr. Zhangs catalysts and scaled its production to multikilogram scale, sufficient for efficient, cost-effective manufacturing processes for metric tons of APIs and intermediates.
Quite often during pharmaceutical development projects, process chemists are called upon to develop several alternate, competitive routes to a given complex API. Development groups then assess each process according to material and energy costs, and settle on the best synthetic route. Critical factors in determining cost are: raw material and reagents, reactor productivity (combination of reaction time and concentration), yield, number of steps, and the need and availability of specialized equipment or technology (e.g., low temperature, high pressure, high-energy reagents). Although introducing the chiral center is a key aspect of costing out a synthesis, it must nevertheless be considered within the context of the total synthesis.
An easily forgotten aspect of chirality introduction is substrate manufacture. For APIs or advanced intermediates, the substrate must, of course, fit into the synthetic scheme, react favorably with the chiral reagent, and be physically available when the catalyst becomes available.
Simultaneous with its catalyst development efforts, Chiral Quest saw the need to devote resources to efficient routes for substrate manufacture as well. One way of addressing this critical need was through a partnership with Chemical Synthesis Services (CSS), a subsidiary of Almac Sciences. CSS provides process research and development for complex, multistep syntheses typically encountered in pharmaceutical process development.
Recognizing the complex set of choices faced by developers of chiral drugs, Chiral Quest and CSS collaborated on several target compounds to explore how asymmetric hydrogenation would fit within the synthesis of important chiral building blocks and APIs.
Chiral Collaboration
This ongoing collaboration is part of a chiral consortium consisting of Chiral Quest, CSS, and IEP (www.biocatalysis.com). The consortiums mission is to facilitate implementation of chiral technologies into pharmaceutical processing. Consortium competencies draw on Chiral Quests asymmetric hydrogenation expertise, enzymatic transformations from IEP, and process development expertise of CSS.
Although most of the projects of this partnership are confidential, a non-proprietary example of one of our projects, the synthesis of Ethyl (R)-2-hydroxy-4-phenylbutyrate, a key chiral intermediate in the manufacture of ACE-inhibitors, illustrates the consortiums capabilities.
A recent review of competitive routes to this building block notes that the chiral center can originate from the chiral pool (basic chiral building blocks), through either conventional or enzymatic resolution, or may be introduced through asymmetric hydrogenation. Several options for the overall process, individual reactions, and choice of substrates are available.
Working in parallel, Chiral Quest and CSS chemists mapped out the alternate routes to the desired product, while keeping in mind patents that precluded certain routes. Subsequently, CSS developed processes for substrate synthesis while Chiral Quest screened potential asymmetric hydrogenation catalysts for yield, product purity, and enantiomeric excess.
During this stage of the project, CSS uncovered five promising routes to three different hydrogenation substrates (Figure 1). All the while, the two teams conducted economic reality checks to assure that the process not only worked, but would be commercially viable.
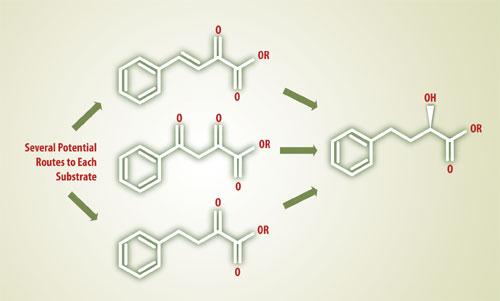
Figure 1: Chiral Quest uncovered 5 routes to three possible intermediates to ethyl(R) -2- hydroxy-4-phenylbutyrate, a key chiral intermediate in the manufacture of ACE-inhibitors
New Technology, Old Molecules
The importance of combining chiral synthesis expertise with process know-how cannot be over-emphasized. By 2007, annual sales of generic pharmaceuticals in the U.S. may reach $60 billion. Of the most recent patent expirations, for drugs approved in the late 1980s, many are chiral compounds. The implications, and opportunities, for chiral specialty companies with process expertise are immense.
Since generic drugs are typically manufactured through a process different from that of the original sponsor, route redesign is becoming a much sought-after area of expertise. The Chiral Consortium has made redesign a top priority through its New Technology for Old Molecules initiative. This moniker may sound trite, but it has a basis in the changing nature of chemical synthesis.
Drugs coming off patent today were originally designed at a time when manufacture-worthy chiral syntheses were rare. Despite the thalidomide experience, it took until around 1990 for the pharmaceutical industry to come to grips with the potential impact of chirality on drugs safety and efficacy.
Introduction of the first chiral switches, previously racemic drugs resynthesized as single isomers, was a major impetus for drug developers to reconsider the significance of chirality.
Some gentle nudging from the FDA, in the form of a regulatory guidance, was helpful in asserting the new chiral business model. FDA began formulating a position on chirality during the late 1980s and published its official guidance on single-isomer drugs in Development of New Stereoisomeric Drugs (1992, last updated in 2005).
The FDA based its position on evolving chiral technology and the recognition that enantiomers may exhibit the complete range of similarity or dissimilarity with respect to safety and efficacy. The agency stressed that racemates were still perfectly acceptable, since the common practice of developing racemates has resulted in few recognized adverse consequences. Although it is now technologically feasible to prepare purified enantiomers, development of racemates may continue to be appropriate.
At the time, processes we now take for granted, like asymmetric hydrogenation, were still in their early stages. Moreover, the majority of process-worthy chiral reagents and transformations, most of which emerged during the past ten years, had not yet been invented.
Chiral Hydrogenation
Asymmetric hydrogenation continues to be the dominant synthetic method for producing chiral intermediates at high yield and in close to 100% optical purity. Over the past 35 years, thousands of chiral phosphine ligands used to manufacture chiral phosphine hydrogenation catalysts have been reported in the literature. These reagents usually consist of a phosphorous atom covalently bound to a chiral scaffold and complexed to an active metal such as ruthenium, rhodium, or iridum.
The metals facilitate hydrogen addition to double bonds. Substrates for asymmetric hydrogenation include di- and tri-substituted alkenes (yielding chiral alkanes) plus imines (amine products) and ketones (alcohols). Other substrates have become popular as well, for example dehydroamino acids, enamides, and enol acetates.
The history of asymmetric hydrogenation parallels that of chiral synthesis in general. The earliest phosphine ligands, DiPamp and Binap, created high expectations for asymmetric hydrogenation in the manufacture of chiral intermediates.
Early on, the patent holders struggled over how best to capture the value of the IP inherent in chiral phosphine ligands. Manufacturers attempted to capture downstream value aggressively, only to experience resistance from process development chemists who simply sidestepped relevant patents by applying what was at the time more cost-effective classical resolution using diastereomeric salts. Interestingly, the main technical hurdle was not the hydrogenation process itself, but development of scalable, cost-effective manufacture of the chiral ligands.
Binap, for example, incorporates a biaryl ligand whose chirality derives from atropisomerismrestricted rotation about a single bond. DiPamp, by contrast, is chiral at phosphorus.
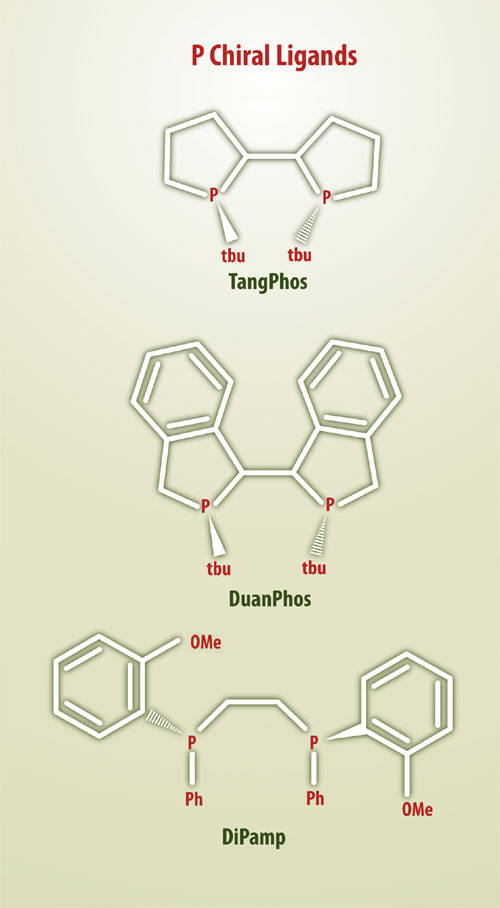
Figure 2. Examples of P-chiral ligands whose asymmetric center is located at phosphorus rather than carbon
P-Chiral Ligands
P-chiral ligands attracted little interest between the early Monsanto work in the 1970s and the discovery, by Dr. Zhang, of a novel family of electron-rich P-chiral hydrogenation catalysts in the 1990s. The first of these was Tangphos, which showed broad applicability but requires a chiral pool base for its synthesis that exists in only one enantiomeric form.
The second-generation electron-rich P-chiral ligand, Binapine is available in both enantiomers. Recently, Dr. Zhang discovered a third generation P-chiral ligand, Duanphos, which is applicable to a broad range of asymmetric hydrogenations, including the reduction of beta-amino ketones. Figure 2 shows the leading P-chiral ligands, including Tangphos and Duanphos, which have been commercialized by Chiral Quest.
A continuing issue in the synthesis of phosphine ligands is the source of the phosphorus atom. Most ligands are derived from a short list of potential sources, including phosphorous trichloride, diphenylphosphine and its salts, and chlorodiphenyl phosphine. Dialkylphosphines are often pyrophoric and difficult to handle.
Although the learning curve for the production and implementation of chiral phosphine hydrogenation catalysts has lasted longer than anticipated, the technology has finally achieved viability at commercial scale. Numerous products are either in full production or at advanced stages of development.
While chiral chemistry has not yet achieved commodity status, the choices and opportunities in chiral chemistry have never been greater, both for pharmaceutical manufacturers and suppliers of chiral technology. We expect that new asymmetric reactions will continue to emerge from academic labs and small specialty chemical companies. The degree to which these technologies find a place in large-scale pharmaceutical manufacturing will depend on how well drug sponsors and vendors integrate chirality into the entire manufacturing process.







