April 1, 2011 (Vol. 31, No. 7)
Over $50B in Product Sales Have Been Posted in the Last 20 Years as a Result of Biotech Advances
Biotechnology stocks were up about 12% over the past 12 months through February 2011, underperforming the Nasdaq, which was up 29%. The NYSE Arca Biotech ETF did better, up 23%. Since the recent market bottom in August, the Nasdaq composite outperformed biotech by more than 12%.
Biotech stocks can catch up in 2011 as many new products are expected to launch. Moreover, the clinical pipeline is robust with more than 2,000 products in Phase II or later and almost 5,000 in Phase I and later. In addition, M&A activity should continue as larger cap companies with strong balance sheets acquire technology and pipeline. One major issue is whether new technologies can address therapeutic needs in a cost-effective manner and obtain HHS and CMS reimbursement.
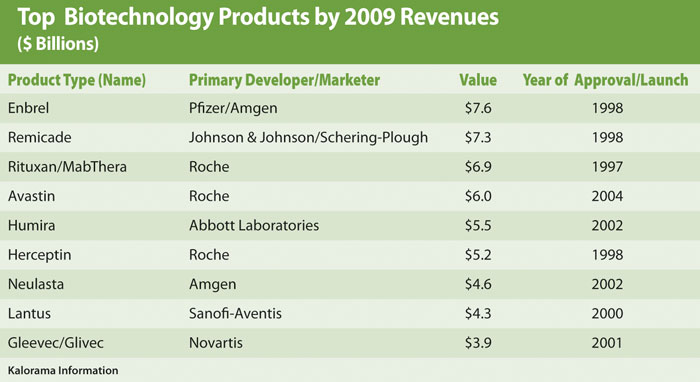
Over the past 20 years, breakthrough technologies in the life sciences have resulted in over $50 billion in new product sales. Monoclonal antibody technology still accounts for most of the multibillion dollar biological products such as Avastin for cancer and Remicade and Humira for rheumatoid arthritis. Data provided by Kalorama Information for 2009 shows that revenue for the top biotechnology products was $51.3 billion (Table).

Rod Raynovich
Of these top biotechnology products, most are indicated for cancer and autoimmune diseases. Over 60 late-stage biotech products have revenue potential of $13 billion in 2015. Recombinant engineered (rDNA) cell lines offer a vast portfolio of products for new vaccines, gene therapy, and monoclonal antibodies with 25 major products expected in the 2011 launch cycle such as Human Genome Sciences’ belimumab for lupus and Amgen’s denosumab for oncology.
Scientific themes and clinical milestones drive investors’ interest in addition to top-line revenue growth. Many emerging technologies are coming on the market in 2011 and will have a major market impact in the intermediate term.
Two very promising technologies that have excited both Wall Street and scientists are antisense and RNA interference (RNAi). After more than ten years and billions of dollars in development, these technologies have had a number of clinical and business setbacks but are still being aggressively funded at about $250 million per year. Two companies that are highly focused in these areas are Isis Pharmaceuticals for antisense and Alnylam for RNAi.
Immunotherapy
A major breakthrough in the treatment of prostate cancer was FDA approval of Dendreon’s Provenge after more than 10 years of R&D and $1 billion in funding. The product is on the market with forecasts in the $375 million range for 2011, with potential peak revenues at $4 billion.
Provenge is an active cellular immunotherapy utilizing the patient’s own cells to elicit an immune response to destroy cancer cells. Additional products are in the Phase I pipeline to treat breast, ovarian, and colon cancers targeting the Her2/neu receptor.
The company recently completed $500 million in funding through a convertible debt offering and is expanding its manufacturing in the U.S and Europe. The cost for Provenge treatment in the U.S. is $93,000/year but reimbursement in Europe may be lower. EU approval is expected in early 2012.
One big cost advantage of Provenge over other cancer treatments such as chemotherapy is that it requires less supportive care to treat side effects. As the drug gains acceptance the focus will shift to cost effectiveness and life extension now pegged as much as 10 months longer than patients not treated.
A September 2010 Kalorama Information report estimates the cancer vaccine market for 2011 at $2.2 billion, it is forecasted to grow to $7.7 billion in 2015 driven by the approval of Provenge.
The major indications for cancer vaccines will be prostate and cervical cancer, with Dendreon expected to dominate the prostate market with 93% share or $5.9 billion in 2015. Cervical cancer vaccines to prevent HPV infection currently on the market—Cervarix (GlaxoSmithKline) and Gardasil (Merck)—are preventive not therapeutic, and are forecasted at $1.8 billion in 2015. This forecast may be low as a higher incidence of HPV is now being reported in males.
OncoThyreon’s Stimuvax therapeutic vaccine utilizes a cancer-associated marker called MUC-1 in a liposomal formulation (BLP25) to create an immune response. Two Phase III trials are under way initially for non-small-cell lung cancer. The product is being funded and developed by Merck KGaA under a license from OncoThyreon.
Geron is in a Phase II trial with an autologous dendritic cell vaccine targeting telomerase in patients with acute myelogenous leukemia.
Vaccinogen has four fast-tracked products in clinical trials using its OncoVAX platform for indications in colon cancer, melanoma, and renal carcinoma. A Phase IIIa trial in the Netherlands demonstrated efficacy with stage 2 colon cancer patients. OncoVAX utilizes the patients own tumor cells patient to create an immune T-cell response capable of destroying cancer cells.
Oxford BioMedica has technologies in gene delivery and immunotherapy. The 5T4 tumor antigen, a unique protein found in many cancers, is utilized in the company’s TroVax therapeutic vaccine currently in a Phase II trial for prostate cancer. An antibody program is also under development.
Stem Cell Therapy
Stem cell technologies are of great interest to investors and scientists because of their purported potential to offer therapies for many diseases. In 2004 California voters gave the go ahead to provide $3 billion in funding for embryonic stem cell research. Seven years later after $1 billion in project funding through the California Institute for Regenerative Medicine no major milestones have been found nor clinical trials initiated.
Many companies have significant funding and several have reached the clinical stage using adult stem cells. Deals and collaborations are de rigueur. Cephalon announced in January that it was acquiring a 20% stake in the Australian stem cell company Mesoblast to develop treatments for cardiovascular and CNS disorders. Mesoblast has an ongoing Phase II trial using its Revascor adult stem cell product against for congestive heart failure. Cephalon plans to invest $350 million into the company including licensing, equity, and R&D milestones.
Henry McCusker, director of research and founder of Scimitar Equity, sees strong investment in R&D. He pegs adult stem cell research at $2.4 billion. McCusker believes the companies to watch are Geron, Cytori, Biotime, Pluristem, and Osiris.
Geron’s ongoing U.S.-based stem cell clinical trial is evaluating pluripotent human embryonic stem cells for treatment of spinal cord injury. Another Geron product candidate is in the preclinical stage utilizing cardiomyocytes for heart disease. The company has spent over $600 million since inception developing IP and its anticancer telomerase technology.
Last year’s financial results showed over $175 million in cash but losses of over $100 million. In the stem cell sector, cash is king as exhibited by the flurry of financings over the last four months. Check balance sheets and burn rates before investing.
Pluristem recently completed at $38 million public offering and has a platform technology PluriX (placental expanded cells) for growing adherent stromal cells that mimic a bone marrow environment. It is proceeding to a Phase II/II trial for critical limb ischemia.
Biotime sells stem cell products for regenerative medicine research and has a good cash position of about $20 million. Cytori, which utilizes adipose-derived and regenerative cells for heart disease and regenerative medicine, raised $20 million in October 2010 and has $60 million in cash (minus Q4 burn), including a $10 million partnership with Astellas focused on liver disease. Revenues are expected to be in the $12–$15 million range in 2011.
Osiris Therapeutics’ lead biologic product, Prochymal, utilizes mesenchymal stem cells from healthy adult donors and is in Phase III trials for acute and steroid refractory graft vs. host disease on an FDA fast track. Prochymal is also in Phase III trials for Crohn disease and acute radiation syndrome. Chondrogen is in Phase II trials for osteoarthritis of the knee. The company has a strong balance sheet with about $75 million in cash.
Advanced Cell Culture
Vaccines are a growth market and more important than ever due to cost effectiveness and prevention of epidemic infectious diseases. Immunostimulants (adjuvants) and improved delivery systems such as nanoparticles can boost the immune response making vaccines more potent. Production of vaccines is moving away from ancient chicken egg production to faster techniques, including mammalian cell culture.
Baxter and Novartis are scaling up manufacturing and expect to get FDA approval for influenza this year. Crucell’s PER.C6 cell line can facilitate large-scale manufacturing of antibodies and vaccines. Crucell was sold to Johnson & Johnson through a $2.4 billion tender offer in February.
Next Generation Protease Inhibitors
Hepatitis C (HCV) is a major health problem affecting 180 million worldwide and 4.1 million in the U.S. Medical costs are in the $30 billion range. Protease inhibitors that block viral replication such as Vertex’ Telaprevir and Merck’s Boceprevir are in the final stages of FDA approval and products should roll out by mid-2011 with worldwide revenue potential of more than $4 billion. Next-generation protease and polymerase inhibitors are also in the pipeline by several companies.
Beyond the next five years we should look at major markets with unmet medical needs where technology is lagging. In a recent report, J.P. Morgan highlighted 20 indications that have a high unmet medical need. The top two—obesity and Alzheimer disease—have a combined market potential in excess of $20 billion with no significant drugs on the market.
Obesity has been a holy grail of drug development for many years with a long history of disappointments. There are at least 10 late-stage clinical trials under way for AD, but it continues to be a difficult disease due to lack of scientific and medical understanding regarding the mechanisms and the need for biomarkers.
Rod Raynovich ([email protected]) is a principal at Raygent Associates.



