March 1, 2014 (Vol. 34, No. 5)
Eric S. S. Langer President and Managing Partner BioPlan Associates
Efficiency and Productivity Continue to Be in Demand According to Recent Survey
Demand for greater productivity and efficiency will be major trends in 2014. According to research and data from BioPlan’s 10th Annual Report and Survey of Biopharmaceutical Manufacturing, the areas of greatest interest this year will likely include greater adaptability, efficiency, and more portability. In fact, a main reason the industry is looking to increase its use of single-use devices is for the flexibility afforded by a “modular” approach.
Our research has also revealed a greater need for:
- Flexibility and modularity
- Adaptability to integrate new technologies
- Clone-ability to duplicate facilities and processes
We’ve also seen growth in outsourcing and off-shoring of more high-value areas. Beyond typically outsourced activities like fill-finish, validation services, and assays, companies are increasingly relying on their suppliers to address more challenging processes.
From expertly packing chromatography columns, to providing higher-quality liquid media and buffers at large-scale, service suppliers with technical competence are taking on more challenging operations. Developing regions, including China, India, and Brazil, are emerging as opportunities, based on their rapidly expanding middle-class population and the need for greater investments in healthcare.
Some of the trends that support efficiency and productivity this year include:
• Fully single-use facilities are on the horizon: Demand for single-use products has grown rapidly, and some disposable devices have almost fully penetrated precommercial manufacture. It may be only a matter of time before they’ll be widely adopted in commercial manufacture, too.
In our study of 235 biopharma decision-makers, almost half (46%) agreed that they expect to see a 100% disposable facility in operation within five years. Capacity bottlenecks in the future can be avoided through more “modularized” production systems that capture the cost, efficiency, product quality, and other benefits of newer technologies. This is reflected in the desire for facilities to be leaner—with cost reductions, increased productivity, and improved quality—and more flexible than their current platforms allow.
• Demand for greater innovation: The bioprocessing industry is entering a more mature phase. As a result, innovations such as continuous bioprocessing, more cost-effective downstream processing, better sensors/probes, and better analytical techniques to permit more biosimilar manufacturing in emerging regions are essential. Innovation that integrates different systems from different suppliers, and a plug-and-play attitude is also emerging within the industry.
• Reducing capital investment in facility and equipment: Efficiency and productivity means doing the same (or more) with fewer resources. For example, our annual study found 26% of the industry reported seeing a reduction in capital investments as the most critical reason for increased use of single-use devices (up from 17% in 2012). Technologies that don’t require a huge up-front investment will have an advantage.
In addition, the desire to avoid being stuck with inefficient, legacy manufacturing systems during a drug product’s life cycle is an increasing concern. The biomanufacturing community does not want to invest too heavily in legacy or inflexible platforms. This concern has led to an interest in processes that are more flexible than certain rigid stainless steel equipment.
• Emerging markets growing: Biopharmaceutical facilities are being built by local companies in developing countries to serve domestic and regional markets. Markets for biopharmaceuticals are growing in many developing countries, such as India and China, with increased incomes, a new middle class, and improved healthcare.
According to our WIKI resource (www.top1000bio.com) on global biopharmaceutical facilities, China (8.6%) and India (8.1%) together account for about one-sixth of global capacity. Add in hubs in other Asian countries (9.7%) and Latin America (6.6%), and emerging regions hold a combined one-third of estimated global concentration of bioprocessing capacity, employment, and productivity.
• Core activities being outsourced: Activities previously deemed too core to be outsourced are now being outsourced. For example, in our latest study, 44% of industry respondents outsourced at least some downstream process development, a significant increase from 29% a year earlier and 22% in each of the prior two years.
Similarly, outsourcing of upstream process development has more than doubled since 2010, from 17% to 43%. Comparable rates of growth are also seen with outsourcing of DoE and QbD services. Despite the growth rate in higher-value outsourced activities, the heaviest levels of outsourcing activity are still in lower-value services. On average, facilities outsource 32% of analytical testing/bioassays, 32% of their total fill/finish operations, and 29% of their toxicity testing.
• Budgets rebounding: Study data and trends point to an industry that is expanding in scope, and with budgets that are also increasing. During the past recession, budgets were virtually frozen. But since 2008, companies are allocating budgets more broadly (albeit more carefully).
For example, over the past five years, respondents to our annual studies have gone from forecasting moderate decreases in spending (of –1.3% in 2009) on outsourced biopharmaceutical manufacturing to +1.7% budget increases in 2013. Hiring of new operations and scientific staff has undergone an even bigger turnaround in budgets during those years—with that swing very likely due to a general loosening of budgets after recessionary staff reductions.
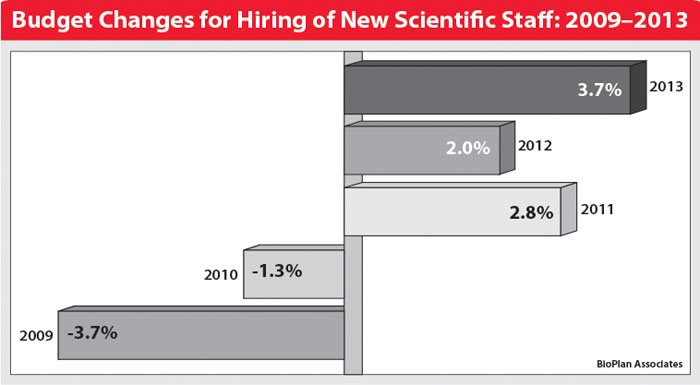
Budget Changes for Hiring of New Scientific Staff 2009-2013
Eric S. Langer ([email protected]) is president and managing partner at BioPlan Associates. He is editor of numerous studies, including “Biopharmaceutical Technology in China,” “Advances in Large-Scale Biopharmaceutical Manufacturing,” and many other industry reports.







