March 15, 2006 (Vol. 26, No. 6)
Label-Free Solutions Designed to Provide High-Quality Data in Real Time
Proteins and their many interactions play a crucial role in biological systems. Detailed characterization of these interactions is important to uncover disease processes and develop therapeutic products.
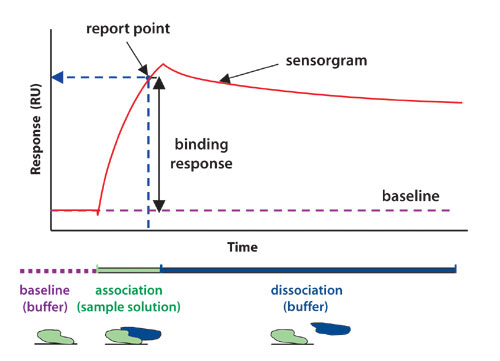
Protein interaction analysis systems from Biacore (www.biacore.com) use the natural phenomenon of surface plasmon resonance (SPR) to deliver high-quality data in real time without the use of labels (Figure). The analysis procedure is straightforward. One of the interacting molecules is immobilized onto the surface of a sensor chip. A potential interaction partner is then injected in solution and flows over the sensor surface. As an interaction partner binds to immobilized molecules, refractive index at the surface alters in proportion to the change in mass concentration. This results in a change in SPR signal (measured in resonance units RU) that is detected in real time and presented as a sensorgram.
Sensorgrams display the formation and dissociation of complexes over the entire course of an interaction, with the kinetics (association and dissociation rates) revealed by the shape of the binding curve. The affinity (strength) of the interaction is determined from steady-state responses or from the kinetic values. A label-free approach eliminates the risk of experimental artifacts, as well as the added costs and safety issues associated with label technologies.
Antibodies
Antibodies and antibody fragments play an increasingly important role in research and drug discovery where they are used to help unravel the details of biological mechanisms. Clearly defining their interaction characteristics ensures that the best antibodies are selected as research tools or assay components. Biacore T100 and Biacore A100 enable the characterization of antibody interactions to be completed within days rather than months. Biacore A100 offers higher productivity with an array-based format that enables interactions to be monitored in parallel. For those requiring rapid, preliminary selection from a large number of samples, a second array-based system, Flexchip, enables up to 400 interactants to be screened against one sample in a single run.
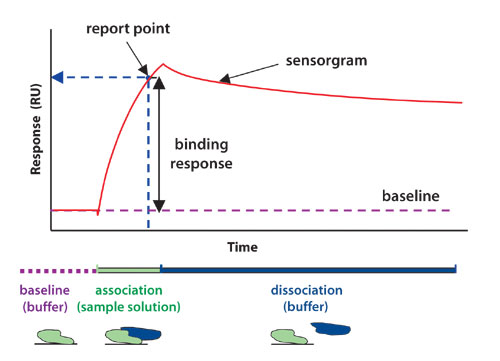
Figure: A sensorgram shows the interaction of a protein with another molecule in real time.
Proteins in Research
Native and recombinant proteins are used extensively from investigations into disease pathways through to the identification of protein targets for potential drug therapies. As with antibodies, Biacore T100 and Biacore A100 offer characterization, and Flexchip a fast analysis, of multiple interactants against a single sample.
For more in-depth studies of a specific interaction, the temperature control, buffer degassing, and evaluation software tools in Biacore T100 are well-suited for investigating the thermodynamics of an interaction. The ability to combine thermodynamic analysis with high-resolution kinetics facilitates accurate, detailed characterization of transition-state formation during association and dissociation phases.
For scientists taking the proteomics approach to study disease pathways and identify potential drug targets, the throughputs offered by Flexchip and Biacore A100 can be an advantage, bearing in mind the large number of samples that are often involved. Biacore 3000 isolates and characterizes proteins for recovery and deposition directly onto MALDI targets for analysis by mass spectrometry, which offers an advantage when working with small amounts of unknown proteins.
Biotherapeutics
Biotherapeutics may take the form of antibodies, antibody fragments, or other recombinant proteins. Biacore A100 enables interaction analyses of multiple samples with multiple interactants, facilitating early selection during screening phases, reducing cost and time, and enabling better decisions to be made regarding candidate selection.
Stable temperature control makes Biacore T100 well-suited for studying interactions at physiological temperature, optimizing the predictive value of the binding data obtained for downstream in vivo testing and clinical use. The high information content data provided by both systems also facilitates critical decisions regarding dose and administration efficacy.
From the earliest stages of discovery through to development and quality control, it is important that laboratory practices comply with regulatory requirements. Biacore T100 and Biacore A100 comply with the FDAs 21 CFR Part 11 Rule and are designed for use further downstream in industry sectors that are subject to strict GLP/GMP/GCP regulatory requirements.
Biacore C is a system dedicated for GxP-compliant, rapid concentration measurements required from preclinical development through to quality control. Results are used to check process control and for batch-release assays.
Immunogenicity
Of growing significance in biotherapeutic development is the safety aspect surrounding their use. Proteins introduced as therapeutics inevitably induce an immune response. Such a response may, at best, reduce the efficacy of treatment and, at worst, lead to the build up a life-threatening immune reaction.
Measuring the antibody response to treatment is now a regulatory requirement during the development of protein therapeutics, and post-marketing surveillance of immunogenicity of protein therapeutics is an FDA requirement. Biacore T100 and Biacore A100 systems detect an immune response by characterizing serum antibodies. Recent results show that the Biacore system detects low-affinity antibodies that may be clinically significant.
Similar immunogenicity studies can be performed during vaccine/immunotherapeutic development, measuring and characterizing antibody responses in serum. Of course, in these studies the build-up of a strong antibody response mechanism is desired.
Small Molecule Drug Candidates
Comprehensive characterization of the interaction of potential drug candidates (typically <1,000 Da) with a target protein is important during the early stages of drug discovery when hundreds to thousands of potential lead compounds must be investigated.
Biacore T100 and Biacore A100 are well-suited to characterize the kinetics, specificity, and affinity of drug candidates with a target protein at these early lead selection stages. Again, the choice of system is based upon the experimental productivity required and, since both systems are designed for GxP environments, methods can be seamlessly transferred through later clinical stages, manufacturing, and quality control.
In Summary
Comprehensive protein interaction analysis increases productivity and cost efficiency throughout research, drug discovery, development, manufacturing, and quality control. Protein interaction analysis with Biacore systems is noninvasive, label-free, and delivers high-quality results in real time.