June 1, 2011 (Vol. 31, No. 11)
Noora Lindholm
Marika Suomalainen
Sini Suomalainen
High-Throughput Workflow Solution Now Available to Obtain High Yields of Pure DNA
The effective purification of nucleic acids from various cell and tissue types is an essential starting point to a broad variety of experimental protocols. With downstream uses in PCR and restriction analysis, for example, purified nucleic acids are essential to different scientific study areas, including drug discovery and medical research. As such, the quality of the starting material—the DNA—is essential.
By using magnetic bead based technology, where magnetic particles are transferred instead of the liquid, residuals are no longer present to decrease purity. As a result, users can extract high yields of pure DNA from cell or tissue samples for downstream use.
In this issue, we discuss the new Thermo Scientific KingFisher Cell and Tissue DNA Kit (Thermo Fisher Scientific), and how it has been used to determine the optimal conditions for maximizing purity and yield from cell and tissue samples, consequently increasing the efficiency of downstream applications. By providing a unique product portfolio including the new kit, the Thermo Scientific KingFisher Flex and BindIt software, an automated process with walk-away capabilities can be produced. Workflows are, therefore, streamlined to enable faster throughput while maintaining high levels of sample purity and yields.
Methods
The DNA-purification process was performed using the KingFisher® Flex, in combination with the KingFisher Cell and Tissue DNA Kit, which consists of: KingFisher Magnetic Beads, Lysis Buffer, Binding Buffer, three Wash Buffers and an Elution Buffer. The magnetic particles are characterized by a high surface area and uniform size distribution, making them suitable for high-throughput processing.
DNA was purified from different sample materials:
- 1 x 106 frozen HeLa-S3 cells
- One punch (0.2 cm diameter) mouse ear
- 0.1–0.2 cm mouse tail
- 15 mg mouse liver
- 15 mg mouse kidney
- 20 mg mouse spleen
In order to assess the best conditions for optimizing yield, different processing times for lysing the tissues were tested, and DNA was eluted in 150 µL of the Elution Buffer
Sample lysis. The DNA-purification process begins with homogenization and lysing of the sample. Efficient lysis is extremely important in order to gain high yields of quality DNA. The sample material must be effectively lysed to enable the magnetic beads to bind to the DNA in a suitable buffer. Subsequent washing steps dispose of inhibitors, and high-purity DNA is eluted into the elution buffer. Optimal lysis time differs between cell and tissue types, due to various cellular and extracellular molecular structures, for example hard tissues such as cartilage and bone.
Lysis of cells. After the addition of the 200 µL Lysis Buffer, the HeLa-S3 cells were efficiently mixed by pipetting and subsequent vortexing for 30 seconds, until the viscosity was lost. The samples were incubated for 15 minutes at 70ºC.
Lysis of tissue samples. Ear, kidney, and liver samples were lysed in 250 µL Lysis Buffer and vortexed for 15 seconds. Parallel samples were then incubated at 56ºC for 15 minutes, 1 hour, 4.5 hours, and overnight. Spleen and tail samples were only lysed overnight (due to limited availability of sample material). Following lysis, all samples were centrifuged for 5 minutes at 6000 x g to separate out the cell debris from the lysate.
The 15 minute lysis of the tissue samples occurred directly in the KingFisher Flex embedded heating block. The samples lysed for longer time periods (1 hour, 4.5 hours, and overnight) were incubated in a benchtop heating block.
Purification. Each sample type was purified using the magnetic bead technology of the KingFisher Flex. This ensures that the final DNA is of high quality and free from proteins, nucleases, and other contaminants or inhibitors.
Once the samples are cleared of any cellular debris, the lysate, Binding Buffer, and KingFisher Magnetic Beads were all transferred to the KingFisher Flex instrument. This technology is based on the use of magnetic rods, which move particles through the various phases of purification (binding, washing, and elution). By transferring the magnetic particles instead of liquids, there is no chance of contaminating residual carryover and a rapid and reproducible protocol is therefore ensured.
During the binding step, the target DNA binds to the magnetic beads, in the presence of chaotropic salts. In order to provide highly pure DNA, the wash steps effectively remove any potentially contaminating proteins or cellular debris. The purified DNA is then eluted from the magnetic beads into the Elution Buffer. The purification process within the KingFisher Flex took approximately 25 minutes per 96 samples.
Results
The data obtained from the purification of various cell and tissue samples demonstrated that high yields of quality DNA are easily extracted with the KingFisher Cell and Tissue DNA Kit.
DNA purified from mouse ear, liver, and kidney samples produced a high yield of DNA, as shown in Figure 1. This is demonstrative of the ability to provide quality starting material for applications, such as the genotyping of mice, where ear and tail are typically used. DNA yields obtained depend on both the lysis time and volume of tissue; 10 mg and 15 mg of mouse kidney samples were lysed for 1 hour, 4.5 hours, and overnight, the results of which are shown in Figure 2.
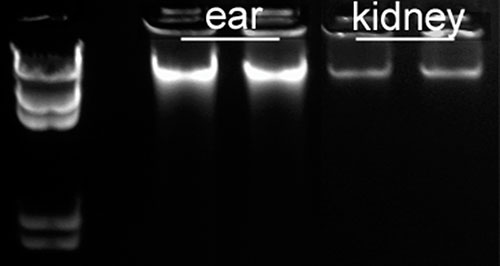
Figure 1. Simultaneous purification was performed from mouse ear, liver, and kidney samples and high levels of DNA were obtained.
However, even after only 1 hour of lysis, the DNA obtained was of an excellent yield and ratio, making it ideal for use in downstream applications. In addition, lysis of the ear, liver, and kidney samples in the KingFisher Flex for 15 minutes was effective, producing an adequate yield for subsequent PCR reactions (data not shown).
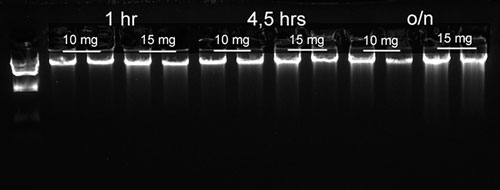
Figure 2. 10 and 15 mg samples of mouse kidney were lysed for 1 hour, 4.5 hours, or overnight, followed by DNA purification.
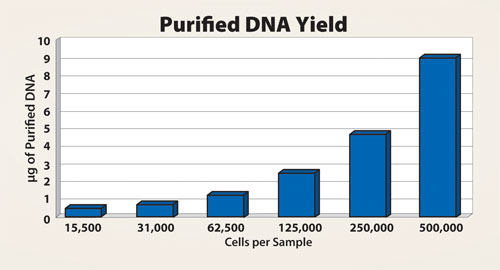
The effects of the amount of sample material were compared with the purification of DNA from a different quantity of HeLa-S3 cells. The results indicate that there is a direct correlation between the yield of purified DNA samples and the number of cells in the sample, as shown in Figure 3. Even with the smallest amount of approximately 15,000 cells, the purification process was efficient and the subsequent PCR was successful.
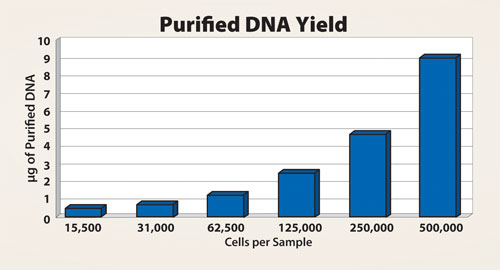
Figure 3. Dilution series from HeLa-S3 cells indicating that the purified DNA yield correlated with the quantity of cells within the sample.
Conclusion
Efficient and fast DNA purification is an essential starting point to a broad array of drug discovery applications. As such, it is essential that researchers have a reliable method of obtaining high yields of pure DNA. The KingFisher Cell and Tissue DNA Kit, in combination with the KingFisher Flex offers a high-throughput workflow solution.
Since different sample materials require a range of lysis times, the entire purification process can be effectively optimized to take into account the volume and type of material being used. As a result, optimal yields are produced from each purification for use in downstream applications. Additionally, with some materials, the hands-on time can be minimized and throughput maximized by performing the lysis directly in the KingFisher instrument.
Noora Lindholm ([email protected]) is product manager, and Marika Suomalainen and Sini Suomalainen are application scientists at Thermo Fisher Scientific.