December 1, 2011 (Vol. 31, No. 21)
Coupling Capillary Electrophoresis to Mass Spec Opens Up Possibilities Not Previously Available
The ever-changing needs of pharmaceutical laboratories are pushing analytical chemists to create better, more efficient characterization methods for their target compounds. Key to this process is the use of separation tools that provide sufficient resolution, speed, sensitivity, and robustness in these rigorous environments.
The most common separation tool in pharmaceutical analytical laboratories is liquid chromatography (LC). LC offers high resolution, flexibility, and a diverse group of chemistries that deliver the ability to separate a broad range of compounds. When coupled to mass spectrometry (MS), LC can provide valuable insight into a compound’s metabolism, modification, and exact composition.
LC-MS cannot, however, cover all compound types, and does not provide good differentiation of highly polar small molecules. Furthermore, when coupled to MS, nanoliter flow LC systems lack robustness.
Capillary electrophoresis (CE) represents an alternative tool that provides high-speed, high-resolution separations that excel at resolving polar-charged analytes. The ability to couple an inherently low-flow technique with MS represents a perfect complement to current LC technology, opening analytical avenues that previously were not easily accessible.
In the past, coupling CE with MS has proven difficult; with the advent of the CESI 8000 High Performance Separation-ESI Module with OptiMS Technology* (Beckman Coulter), the barriers to using this technique have been removed. This was accomplished by focusing on the interface between CE and MS.
CE should have a perfect marriage with MS if its inherent advantages of low flow and high resolution are maintained. For this reason, we selected an elegant and simple sheathless interface design. To execute this, a hydrofluoric acid-etched tip was utilized to create a porous region at the capillary outlet.
Figure 1 highlights each of the critical components of this porous sprayer: polyimide-coated silica separation capillary, porous segment, stainless-steel needle, static conductive liquid, and background electrolyte (BGE). Several features of this design make it ideal for this interface.
The capillary and sprayer are one complete piece, meaning there are no junctions or dead volumes. The capillary is not tapered as is common in nanospray sources, thus providing a robust system that does not clog.
This design neatly answers the CE-MS challenges posed by earlier designs.
- Sensitivity—provided by ultra-low flow, even in the mass-sensitive range, with no make-up liquid needed.
- Robustness—an open-tube separation column that maintains a constant inner diameter eliminates clogging common to conventional nanospray interfaces.
- Resolution—CE peak/spike shape is maintained by direct bulk-flow into MS without the dilution associated with sheath-flow designs.
- Electrochemical artifacts—the low ESI voltages needed to reduce oxidation/fragmentation en route to the MS inlet.
To test the capabilities of this system for the analysis of interesting pharmaceuticals, a forensic case was used as a benchmark. It is well established that there is an ongoing requirement in forensic casework to detect and quantify low levels of potent drugs and their metabolites. This need arises especially when there has been an extended period of time between administration of the drug and collection of the sample.

Figure 1. Sheathless porous ESI interface schematic
Forensic Case Study
In this work, fentanyl, and its metabolite, norfentanyl, were analyzed at challenging subtherapeutic levels using capillary electrophoresis coupled via the new electrospray interface to a Waters Xevo tandem mass spectrometer (CESI can interface with a number of mass spectrometers). Fentanyl (Figure 2), a potent synthetic narcotic analgesic, surgical anesthetic, and recreational drug, is commonly administered at low dosages of 25 µg via patch, resulting in the very low therapeutic levels of 0.3 to 1.2 ng/mL in serum after 24 hours.
Samples were externally prepared along with blind controls and analyzed in duplicate in an automated process. Stock solutions were prepared at a concentration of 1 mg/mL and were further diluted for spiking serum with methanol. Standard solutions for mass spectrometry and extractions were prepared at 5, 1, or 0.1 ng/µL in 5 to 50 mM ammonium formate (pH 2.85). The results for both fentanyl and norfentanyl showed excellent linearity (R>0.995) over a range of 0.1 to 50 ng/mL of serum with limit of detection (LOD) and limit of quantitation (LOQ) values of 0.1 ng/mL.
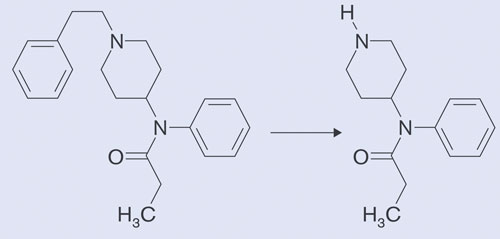
Figure 2. Fentanyl metabolism in biological fluids
Serum calibration samples for fentanyl and norfentanyl using doxapram as the internal standard were prepared and analyzed using a liquid-liquid extraction protocol.
The CESI required an injection volume of 7 nL of extract dissolved in 100 µL. Multiple reaction monitoring was used in the quantitative processing (Fentanyl: 337.4→188.2, Norfentanyl: 233.3→84.1, Doxapram IS: 379.4→292.4). Calibration curves were linear (R2>0.99) for both fentanyl and norfentanyl from 0.1 to 50 ng/mL (data not shown).
LOD and LOQ calculations showed that the protocol can provide detection of fentanyl and norfentanyl at low picogram per milliliter levels in serum (Figure 3). This data was derived from the injection of just 7 nL of material onto the capillary.

Figure 3. Calculated LOD and LOQ
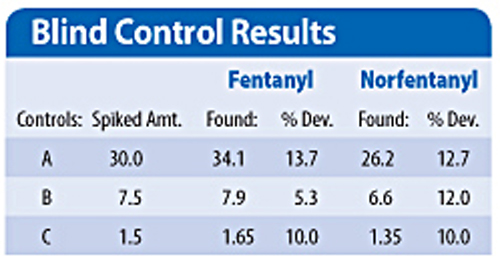
In order to evaluate the technique with unknowns, samples including blanks were prepared by an external agency and extracted in duplicate, bracketing the blind controls. The results for the externally prepared blind controls compared to the target values were acceptable at less than 15%.(Table)
These results confirm that the interface is capable of providing the necessary sensitivity, robustness, and precision for a challenging, qualitative, and quantitative pharmaceutical drug analysis. Very low sample volumes (7 nL in this work) are required compared to existing technology such as LC-MS, making CE-MS using the OptiMS interface virtually a nondestructive sampling technique. This is significant for precious or small, lab-scale syntheses, in which researchers might want to perform multiple analyses on a single reaction.
The sensitivity improvements offered by CESI-MS would permit the analysis of biofluid volumes in the 50 to 100 µL range. By dissolving the residues in less than the typical 100 µL volumes, volumes down to 1 µL can be sampled.
CESI with the Opti-MS interface for coupling capillary electrophoresis to mass spectrometry has been shown in this work to provide the sensitivity required to analyze one of the most challenging drugs, fentanyl and its metabolite, norfentanyl. The small injection volumes required, in this case 7 nL, create a significant advantage for forensic scientists that very often deal with inadequate specimen submission.
This important advance in detection technology permits replicate quantitative analysis on less than 100 microliters of biofluid and detection of many challenging drugs and their metabolites at subtherapeutic levels. Generating stable ESI at flow rates as low as 10 nL/min provides a substantial increase in sensitivity through improvement in ionization efficiency and a reduction in ion suppression. The process is robust and automated and will be of great value to analytical laboratories that work with charged analytes.
Thomas Ed Horton, Ph.D. ([email protected]), is a global marketing manager for analytical products, and John C. Hudson ([email protected]) is a staff scientist for capillary electrophoresis and mass spectrometry with Beckman Coulter. The authors would like to acknowledge Gordon McKay for providing the standards and spiked samples for the fentanyl/norfentanyl study. *In development. For laboratory use only; not for use in diagnostic procedures. Beckman Coulter and the stylized logo are trademarks of Beckman Coulter and are registered in the USPTO. CESI and OptiMS are trademarks of Beckman Coulter.



