October 15, 2013 (Vol. 33, No. 18)
Diverse factors account for the interest in (and success of) the adult stem cell field in recent years.
These factors include breakthroughs in reprogramming, ethical and regulatory issues more tractable than those encountered with embryonic stem cells, and (with a wink to The Graduate) … plastics. Diverse factors will likely contribute to the field’s future as well. At the “Adult Stem Cell Therapy and Regenerative Medicine Conference” factors such as manipulation, transduction, visualization, and homing were considered, as was cell culture.
How the cells are nurtured can be as important as how they are deployed. When it comes to preparing cells for possible (re)introduction into animals, every precaution needs to be taken—from the obvious (keeping them sterile and cross-contamination-free), to the subtle (housing and manipulating them in conditions that mimic their inherent physiology). Mimetic culture can support viability as well as promote the desired differentiation state.
It’s not enough to keep cells in a 37°C, 5% CO2 incubator, passage them in a laminar flow hood, and then view them on a benchtop microscope. According to Kevin Murray, director of sales and marketing for BioSpherix, cells should be maintained in uninterruptable conditions: “Whatever conditions [you use for] culturing your cells…you should also use for processing them. For instance, incubators are set up to mimic body temperature and control for CO2, which is basically how they manage pH in the media. Another aspect we advocate is oxygen control, because cells in the body don’t see room air.”
Researchers generally take great care to match the body’s physiological conditions in their incubators, but the one thing that hardly anybody controls for is oxygen. “And oxygen is tied to the expression of a multitude of genes, to cell signaling—many of the things that researchers ultimately are looking at, whether they know it or not,” Murray said.
While the cells may not venture out of the incubator for very long, many of these genes may be very oxygen-labile—taking hours to upregulate but literally minutes to downregulate. BioSpherix’ Xvivo system is a set of equipment modules—from hypoxic incubators to containment hoods to processing chambers that can house microscopes, centrifuges, and a variety of other equipment, including automation and robotics, all in a controlled environment. The barrier/isolator modules are designed to fit together in a range of configurations to maintain a constant environment in which cultures can be manipulated without being exposed to the room’s atmosphere.
Only a very small percentage of the millions or billions of cells carefully grown and expanded ex vivo and introduced into an animal will survive to engraft and replicate, Murray said. “One of the main reasons they die is that they get very used to room air—about 21% O2—and when you inject them into the body of an animal they’re probably in an environment that’s less than 5% O2.” Mountain climbers need to acclimate to the atmosphere of Mount Everest, Murray pointed out, and something similar is likely true for injected cells.

Closed hoods with integrated incubation chambers are a key design feature in the Xvivio system. According to BioSpherix, incubators open only into aseptic space, and contamination risk is reduced. The hood is controlled for various cell parameters, the same as with the incubator. When cells are removed from the incubator, they experience no disruption in temperature, CO2, pH, O2, etc. Technicians work in soft, flexible, clear plastic-gloved windows, isolated from cells.
Mimetic Cultureware
Growing and preparing cells poses a variety of requirements. For adherent cells, such as mesenchymal stem cells, a key requirement is something that not only supports their ability to attach and spread out, but also allows them to maintain their functionality. Much of this requirement used to be met by biologically based supplements to the culture medium such as serum. As researchers moved toward “leaner” media, serum was often replaced with surface coatings such as fibronectin.
“Our customers that are expanding stem cells for clinical and preclinical use would rather not use the animal- or human-derived material,” explained Paula Flaherty, technology manager, life sciences at Corning. These can be a source of pathogens, and they sometimes require additional screening to demonstrate that they are pathogen-free. In addition, there can be batch-to-batch variability both in the raw material and in the coating protocol. Accordingly, there is growing demand for defined, animal-free culture environments, including synthetic extracellular matrices (ECMs).
Flaherty discussed advances in developing integrated solutions—including media, surfaces, and vessel design—to replicate the functionality of naturally derived proteins. For example, the Corning® PureCoat™ ECM Mimetic cultureware is coated with covalently attached, chemically synthesized collagen I or fibronectin peptides. The company also offers Synthemax®, a synthetic vitronectin peptide-based product specifically designed for human pluripotent cell culture and neuronal cell culture, which is sold as coated plates and polystyrene microcarrier beads, and is available lyophilized to allow users to coat their own culture vessels and glass slides.
Mesenchymal stem cells from various sources including bone marrow and adipose grown on ECM Mimetic or Synthemax in serum-free media showed equivalent population doubling, attachment, tri-lineage differentiation potential, karyotype, and functionality to those grown in standard tissue cultureware in serum-based medium.
That equivalence can also be achieved for stem cells using a combination of Corning’s CellBIND charge-based surfaces and Cellgro serum-free, xeno-free, defined medium, Flaherty said.
Flaherty also emphasized that the ability to scale-up production is crucial to meeting the needs of cell therapeutics. Many of the synthetic (and charged) surfaces are available from 24-well plates up through a variety of sizes of single- and multi-layered T flasks. In addition, using Synthemax microcarriers in scalable spinner flasks “combines the best of both worlds of adherent culture but in a suspension mode so you can get much higher volumes of cells, at much greater density,” Flaherty said.
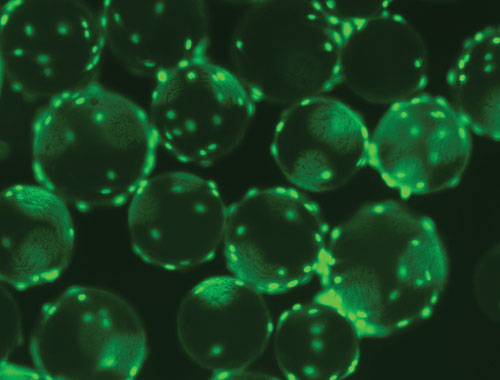
The surfaces of polystyrene microcarriers may be treated to enhance attachment of cells, such as mesenchymal stem cells, to increase cell yield and viability. For example, Corning Microcarriers may be coated with the company’s Synthemax substrate, a synthetic product designed for human pluripotent cell culture and neuronal cell culture.
Lentiviral Reprogramming
Cells used for therapy generally aren’t just taken from a donor, grown, and given to a patient. Typically they’re genetically manipulated in some way to give them their desired properties, whether that means replacing a defective gene, conveying pathogen resistance, or making the cells able to withstand otherwise toxic treatments. Lentiviral technology, based on HIV, is the most efficient system known for reprogramming cell function, said Boro Dropulic, Ph.D., founder and CSO of Lentigen.
Dr. Dopulic recalled that in 2003, when he was still CSO of VIRxSYS, he led the team that first used lentiviral vectors in human trials. He presented a short overview of the field as it has progressed from that groundbreaking work through more recent, promising data coming out of current lentiviral clinical trials.
The VIRxSYS trial utilized a replication-deficient virus with an antisense HIV payload to target HIV-1. “To test HIV vectors in the very first instance it was appropriate to test in an HIV patient, because we really needed to know the outcome of the interactions between an HIV vector and a wild-type virus. Because sooner or later, even if you’re treating patients with cancer or another disease, [you’ll encounter] a patient infected with HIV,” Dr. Dropulic explained. “So it was important to establish the safety in a best case/worst case scenario.” That Phase I trial of five enrolled patients demonstrates the safety of the lentiviral vector.
Others have now followed in their tracks, Dr. Dropulic said, including “the first lentiviral vector trial getting clinical success.” This trial, run by Drs. Patrick Aubourg and Nathalie Cartier in France, showed that when stem cells transduced with a lentiviral vector expressing the ABCD1 gene were introduced into patients with X-linked adrenoleukodystrophy, the patients were able to derive benefits equivalent to hematopoietic cell transplantation, the standard of care. Another project, for β-thalassemia, was so successful that the formerly anemic recipients had to be periodically phlebotomized.
Among other projects, Lentigen is currently involved in a Phase I trial for glioblastoma. The standard of care for this type of brain cancer is surgical resection followed by the chemotherapy agent temozolomide.
“The problem with temozolomide is that with multiple rounds of treatment the drug is toxic to the hematopoietic stem cell compartment, so we’re genetically modifying these cells with the MGMT gene in order to make them resistant to the effects of temozolomide,” Dr. Dropulic explained. “We’ve seen in our clinical trial … that when you transduce these cells with this MGMT gene you actually can increase the dosing for these patients from an average of one and one-half cycles to five cycles.”
The question of where exactly in the body stem cells go after they’ve been injected has never been easier to answer, at least in a mouse.
BioInVision has developed an automated system combining a mouse-sized crytotome with a fluorescence (and bright-field) microscope, allowing fluorescently labeled stem cells to be visualized anywhere in the mouse with single-cell sensitivity. The CryoViz™ system will make 40 µm-thick serial cross-sections, “and then we have a microscope that is moved with a robot over the face of the tissue, getting microscopic images of basically the entire mouse,” said David Wilson, Ph.D., BioInVision’s CTO.
Researchers will use the system to look at the biodistribution of stem cells throughout the mouse. Or in the case of myocardial infarction, stem cells may be used to help treat the injury, and CryoViz will be used to evaluate the homing of cells to the infarcted region.
It takes about 15 hours to image an entire mouse. “The stem cell analysis is automated, and it will run by itself. The next day you can have the 3D images,” Dr. Wilson said. “Then if people want counts of cells in different organs, or densities, we have software to make those analyses as well.”
It can be used to image several fluorescent or quantum-dot reporters simultaneously, and it can be combined with vital imaging techniques such as MRI, PET, and bioluminescence. “We use advanced computer algorithms to do the registration … without the need to add extra fiducials (on the arms and legs, for example),” Dr. Wilson said.
The instrument can be purchased from BioInVision or used by means of a fee-for-imaging service, simply by shipping the company the frozen mouse. Using a tape-transfer technology, researchers can also pick up an entire section and treat the fresh-frozen, non-fixed tissue with a histology stain or labeled antibody for immunohistochemistry. “Say in a mouse we do this with 10 or 15 different sections. The advantage is that we know exactly where that section is within the 3D anatomy,” Dr. Wilson said.
Biodistribution of Stem Cells
Selecting Homing-Ready Stem Cells
Sometimes getting a stem cell to home is a matter of getting it close enough to find the homing beacon. When tissue suffers an ischemic insult—as does the penumbra of tissue surrounding a myocardial infarction—it starts to release the signaling proteins stromal cell-derived factor 1 and vascular endothelial growth factor. These are potent attractors of CD34+, CXCR4+ stem cells, which can help prevent apoptosis, trigger angiogenesis, and preserve the tissue. In the absence of such help, the peri-infarct region is more likely to die in the coming weeks or months.
The problem is that thet stem cells are not hearing the call for help, according to Jonathan Sackner-Bernstein, M.D., vp for clinical development and regulatory affairs at NeoStem.
The company began enrolling patients in January 2012 for Phase II clinical trials of AMR-001, its autologous CD34+, CXCR4+, bone-marrow-derived stem cell product. “We select those cells from the bone marrow, we then administer them into the coronary artery, where they’re smart enough to detect the region of the muscle where they’re needed,” said Dr. Sackner-Bernstein. They move through the walls of the coronary artery into that part of the myocardium and stimulate the development of the blood vessels that the heart is asking for.”
These are cells that are merely selected, not stimulated and expanded in culture. “Once you stimulate cells, we don’t know nearly as much about what they’re going to do and how they’re going to behave when put back into the body,” said Dr. Sackner-Bernstein. “By selecting we’re able to create a product to administer that has a known purity.”
Snag-Free Induction of Pluripotency in Adult Cells
While attractive, the idea of reprogramming adult cells to produce embryonic-like stem cells has not been implemented with much satisfaction. Reprogramming has remained frustratingly slow and inefficient, and it results in stem cells that have limited utility. But now a key impediment to reprogramming has been recognized. Its removal, according to an investigation described recently in Nature (“Deterministic direct reprogramming of somatic cells to pluripotency”), not only shortened reprogramming time by several orders of magnitude, it also improved the efficiency of the process—all the treated cells attained a stem-cell-like state, and they all did so, conveniently, at the same rate.
Adult stem cells may be reprogrammed by inserting four genes into their DNA, a process that yields induced pluripotent stem cells (iPSCs). The process, however, is fraught with difficulty. It can take up to four weeks, the timing is not coordinated among the cells, and one percent or less of the treated cells actually end up becoming stem cells. Success rates can be even lower—around a tenth of a percent—if stem cells are to be used in patients. In such cases, viral gene insertion techniques must be shunned for safety reasons.
The question of efficiency was taken up by researchers at the Weizmann Institute of Science who were already investigating the natural pathways of embryonic development. In particular, researchers in the laboratory of Yaqub Hanna, M.D., Ph.D., asked: What is the main obstacle—or obstacles—preventing successful reprogramming in the majority of cells?
In his post-doctoral research, Dr. Hanna had employed mathematical models to show that a single obstacle was responsible. The identity of the obstacle, however, remained unclear. Then, scientists in Dr. Hanna’s laboratory looked at a certain protein, Mbd3. This protein, a core member of the Mbd3/NuRD (nucleosome remodelling and deacetylation) repressor complex, had caught their attention because it is expressed in every cell in the body, at every stage of development.
Such a protein is quite rare. In general, most types of proteins are produced in specific cells, at specific times, for specific functions. The team found that there is one exception to the rule of universal expression of this protein—the first three days after conception. These are exactly the three days in which the fertilized egg begins dividing, and the nascent embryo is a growing ball of pluripotent stem cells that will eventually supply all the cell types in the body. Starting on the fourth day, differentiation begins and the cells already start to lose their pluripotent status. And that is just when the Mbd3 proteins first appear.
The researchers showed that removing Mbd3 from the adult cells can improve efficiency and speed the process by several orders of magnitude. Efficiency approached 100% from mouse and human cells, and the time needed to produce the stem cells was shortened from four weeks to eight days. As an added bonus, since the cells all underwent the reprogramming at the same rate, the scientists will now be able, for the first time, to actually follow the process step by step and reveal its mechanisms of operation.
Recalling how his team’s discovery was based on research into embryonic development, Dr. Hanna said, “Scientists investigating reprogramming can benefit from a deeper understanding of how embryonic stem cells are produced in nature. After all, nature still makes them best, in the most efficient manner.”
Dr. Hanna’s laboratory is conducting several investigations into iPSCs. These include deciphering the mechanisms of epigenetic reprogramming and induction of pluripotency in somatic cells, including fibroblasts and lymphocytes, as well as the development of iPSC-based experimental systems for in vitro modeling of human disease and development.



