July 1, 2014 (Vol. 34, No. 13)
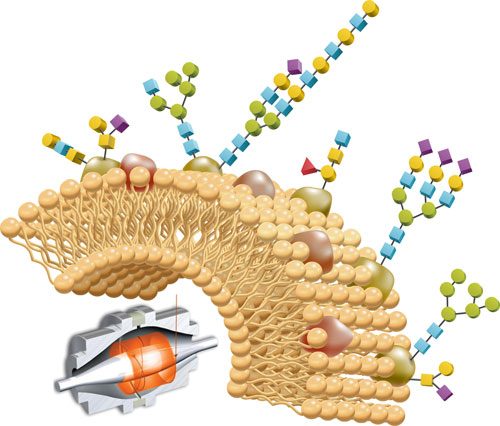
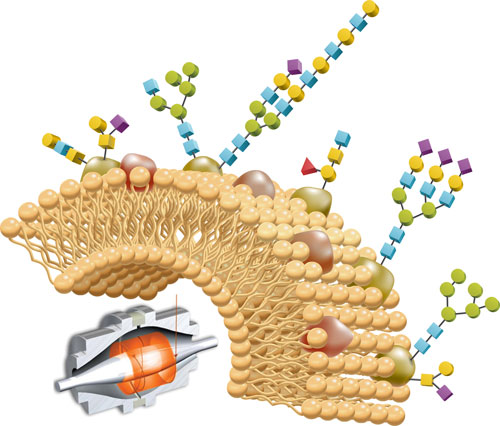
Producers of biopharmaceuticals are finding that the assessment of critical quality attributes is increasingly a matter of evaluating post-translational modifications (PTMs), especially glycosylation.
One such producer, the contract manufacturer Fujifilm Diosynth Biotechnologies, treats PTMs as indicators of process and product consistency. In fact, Min Zhang, Ph.D., the company’s principal scientist for upstream process development, uses the terms “quality” and “PTM” nearly interchangeably.
Bioprocessors and contractors such as Fujifilm have a wealth of experience with PTMs, at least in certain contexts—late in development and through manufacturing. Data, however, is scarce in other contexts—preclinical and early clinical trials.
“In many cases, we are limited to using knowledge of PTMs from a generic antibody as the standard,” admits Dr. Zhang. One example consists of high-mannose glycans, which are associated with very short clearance. As experience accumulates, desirable molecular attributes are based more on the specific molecule and its performance in studies.
The focus on PTMs throughout development underscores the iterative nature
of process development. Developers know which PTMs, particularly glycosylations, are associated with desirable and undesirable attributes, and appropriately try to overexpress or eliminate them.
Dr. Zhang counts protein folding/misfolding and aggregation as independently significant PTMs, even though process conditions and not the usual cell-derived post-translational factors may be responsible. Immunogenicity caused by aggregation is one of the major factors influencing late clinical-stage failures.
Upstream processing, including culture conditions, media, and feed, are known to influence PTMs, but no formula exists for dialing in quality attributes. Process interventions are, as Dr. Zhang puts it, “the million dollar question,” as almost any upstream factor affects quality.
“That includes molecule design, cell line, and process conditions.” Even such pedestrian values as dissolved oxygen and pH are relevant. There is no single way to guarantee quality attributes within spec, but Dr. Zhang assures that “we have many different tools available.”
The Glycan Is the Process
As a testing lab, Eurofins Lancaster Laboratories analyzes therapeutic proteins for such PTMs as deamidation, methionine oxidation, glycosylation, and to a lesser degree phosphorylation. Eurofins obtains glycan profiles by fluorescently labeling them and analyzing by high-performance liquid chromatography using a hydrophilic interaction liquid chromatography (HILIC) column under an organic-to-aqueous gradient, and confirming identity by mass spectrometry. “Fluorescence detection provides high sensitivity,” asserts John Snyder, Ph.D., who works at the company as principal scientist and group leader, proteomics.
Fluorescence labeling is required because sugars lack an ultraviolet chromophore, but tagging adds a step to the analysis. Label-free analysis is possible as well. For example, Agilent’s mAb Glycochip analysis system consists of a microfluidic chip incorporating sample introduction, cleavage of N-glycans by the enzyme glycopeptide N-glycosidase, microchannel ion exchange separation, and direct injection of fractions into a time-of-flight mass spectrometer. Glycans are identified based on matches to a built-in database.
The strength of methods based on initial glycan cleavage is rapid determination of glycan composition and comparison with a reference glycosylation pattern, which is useful for quality testing. The drawback is lack of information on glycan location. For that type of analysis, investigators first subject the protein to protease digestion, followed by mass spectrometry analysis of the fragments for mass additions typical of glycans. Analysis is simplified by knowing what glycans are present to begin with, and what fragments result from proteolytic digestion.
Despite the intermolecular variability in glycosylation, even within a batch, aggregate glycan analysis is a powerful measure of quality assessment. Out-of-specification batches suggests that something has gone wrong with the process, usually upstream.
Mass Spectrometry Approaches
Glycan analysis may be approached from the top down (peptide/amino acid plus glycan) or the bottom up (free glycans). Softly ionizing mass spectrometry techniques are useful in both cases. LAESI (laser ablation electrospray ionization), a softly ionizing mass spectrometry technique, is useful for both.
LAESI involves two steps. First, an infrared laser tuned to the absorption band of water pulses and vaporizes the sample. Second, the sample is carried to a chamber for electrospray ionization. LAESI is an ambient ionization technique, meaning it does not require high vacuum and in many cases samples may be introduced with little or no sample preparation. As with conventional electrospray, samples may be multiply charged. The method was discovered in 2007 at George Washington University and commercialized by Protea Biosciences.
For the bottom-up method, glycans are first released enzymatically in 18O-labeled water. This has the effect of labeling glycosylation sites with that isotope, to distinguish those from deamidation sites that do not take up 18O.
IgGs, the most common therapeutic antibodies today, contain 3–5 kDa of carbohydrate consisting of 3–10 isoforms that predominantly use four or five sugars. Glycoforms differ in terms of number and spacing of sugar residues, sometimes often near the ends of branched chain glycans.
“Today’s high-resolution accurate mass spectrometers can easily distinguish among intact antibody glycoforms, which may differ in molecular weight by as little as 162 daltons,” says Greg W. Kilby, Ph.D., director of molecular imaging and bioanalytical services at Protea Biosciences. That molecular weight difference signifies an extra hexose, for example.
LAESI can also assist in sequencing glycans, similarly to picking apart a peptide or protein. “But this requires a three-stage mass spectrometry analysis, or MS3, usually with an ion trap or Orbitrap,” Dr. Kilby advises. Sequence is often less important than sugar composition, a quality attribute of great interest in biosimilars. This value is accessible through tandem mass spectrometry (MS/MS, or MS2) methods.
Simplest Is Sometimes Best
Perhaps the simplest approach to analyzing glycans involves lectin arrays. Lectins are plant-based proteins that bind specific glycans, for example, those containing fucose or mannose. Since lectins selectively attach to sugars, glycan arrays perform glycan analysis on intact glycoproteins.
“It’s a rapid but somewhat crude technique,” says Peter Sondermann, Ph.D., CSO at SuppreMol, an early-stage biopharmaceutical company. “The technique works especially well for antibodies because you have only one N-glycosylation in the Fc fragment, and only a limited variety of glycans.”
Antibodies could theoretically accommodate many more than the eight or nine glycans they typically hold, but the glycosylation sites are physically inaccessible to the various transferases and glycosidases. By contrast, erythropoietin, the red blood cell-enhancing protein, is much more heterogeneous with respect to glycan content.
Lectin profiling has the advantage over more complex liquid chromatography–mass spectrometry methods in its ability to identify, to some degree, the stereochemistry or regiochemistry of attachment of various sugars. Where the mass spectrometer sees only “galactose” irrespective of the sugar’s location, binding within a lectin array depends on where the sugar is situated.
As glycan analysis improves, so does understanding of how glycan engineering can improve biopharmaceuticals. For example, SuppreMol’s clinical pipeline includes SM101, a soluble Fc gamma receptor IIB (sFcγRIIB), which competes with FcγRs expressed on immune cells for pathogenic immune complexes. Interfering at this stage of the immune response prevents the cascade leading to inflammation and tissue damage.
Previously, Dr. Sondermann worked at GlycArt, which was acquired by Roche in 2005. GlycArt, which specialized in glycoengineering, developed a technology, called GlycoMab, that improves the potency of antibody drugs, particularly toward antibody-dependent cellular cytotoxicity (ADCC), by redesigning the carbohydrate (PTM) component of monoclonal antibodies.
One such strategy involves afucosylation —the depletion of fucose residues from glycans that results in up to 100-fold improvement in affinity of antibodies to natural killer cell mediators of ADCC. This strategy led to the eventual approval of Roche’s Gazyva, approved in October, 2013 in the United States as a first-line treatment for chronic lymphocytic leukemia, and recommended for approval in Europe in May 2013.
QA/QC Is Still Up for Grabs
Demand for glycan analysis has exploded in recent years, fueled by the numbers of monoclonal antibody clinical candidates and advances in automated cell culture development, product recovery development, and formulation screening. But the main driver has been a keener appreciation for controlling critical quality attributes—glycosylation—by varying process parameters. Regulators and manufacturers of biosimilars will closely scrutinize glycosylation patterns to determine the degree of “biosimilarity.”
This all-important QA/QC component of the glycan analysis market is still up for grabs, according John Rontree, director of pharma and biopharma marketing at Thermo Fisher Scientific. This prize is still available even though the company enjoys a commanding position in proteomics. (Thermo Fisher claims that it has supplied 90% of the installed mass spectrometers in this segment.) “The FDA regulations are still unclear on this subject,” Rontree says.
Thermo Fisher has formed a consortium with Amgen and other top companies to clarify the regulatory uncertainty around existing glycomics analysis. The ultimate goal is validated methodologies that the FDA will accept. Rontree notes that despite his firm’s “huge footprint in academic proteomics,” glycomics territory is still wide open.
Among Thermo’s verticalized strengths in molecular analysis is its OrbiTrap Fusion™ mass spectrometer. Orbitrap allows investigators to execute MSn by trapping ions after the first mass spectrometry analysis, and repeatedly subjecting them to fragmentation, over and over, for as many iterations as needed.
Orbitrap Fusion is suitable for both top-down and bottom-up glycan analysis. Conventionally, this analysis involves mass spectrometry of the intact protein, then examining fragments and matching them to database searches. OrbiTrap Fusion can remove one sugar at a time.
The instrument’s only weakness is an inability to differentiate natively between two ends of a branched glycan consisting of different sugars. But for these situations, a combination of glycan-specific high-performance liquid chromatography and inducing larger fragments usually provides the answer.
Thermo Fisher’s Orbitrap Fusion mass spectrometer makes it possible to conduct studies that were unimaginable just a few years ago, including full characterization of an entire glycan structure, large-scale glyco-proteomics, and quantitative analysis.
Chromatography Resins for PTMs?
Protein A chromatography may be the gold standard for capturing monoclonal antibodies, but affinity resins for nonantibody proteins are rather uncommon. Avitide, a New Hampshire-based startup, has developed affinity technology applicable to recombinant proteins, bi-specific antibodies, recombinant vaccines, and some of the more exotic PTM impurities. Many undesirable transformations carry through with the product because preparative chromatography cannot always remove them.
“Protein A serves purification of monoclonal antibodies quite well. It has drawbacks, but the remaining therapeutic proteins are highly underserved in terms of affinity purification,” says Kevin Isett, Ph.D., who founded Avitide. Product-specific chromatography media take too long to develop, and most resins lack robustness for scaleup.
Customers bring Dr. Isett a target, and his firm delivers prototype-scale affinity resins. For production scales, Avitide partners with much larger resin companies. “We can integrate resin development within bioprocess timelines,” Dr. Isett notes.
Apropos this article, Dr. Isett claims success at separating such PTMs such as truncations, deamidations, and pyroglutamate formation that pose huge problems for downstream processing due to their close chemical resemblance to product. Some may adversely affect product quality, potency, and effectiveness by their presence, or because of adjustments downstream processing must initiate to remove them.
Dr. Isett would not divulge the secret behind his success, except to say that he employs “proprietary, high-affinity scaffolds and ligands.”