June 1, 2012 (Vol. 32, No. 11)
China and India Pose Fierce Competition to U.S. Biopharma
The U.S. may still be the world’s superpower, but its dominance in biopharma is eroding as China and India add more innovation to their booming life science industries. The result augurs years of growing competition for biopharma enterprises, and their jobs, just as all three nations are looking to the industry for future growth.
To be sure, the U.S. continues to enjoy the world’s largest biopharma industry by establishments (48,059; Battelle); public-company revenues ($61.6 billion in 2010; Ernst & Young); size of prescription-drug market ($307 billion; IMS); jobs (1.42 million; Battelle); venture capital ($4.733 billion last year; MoneyTree Report); R&D research (just over $100 billion in private and public spending); and patents (6,601 within classes 424, 435, and 514 last year; USPTO).
So Uncle Sam remains biopharma’s anchor—or more accurately, two anchors. California and Massachusetts are the top states in biopharma jobs, companies, venture capital raised, NIH grant funding awarded, and patents granted.
But India and China have grown faster. Both emerged more than a decade ago as offshoring havens driven by lower wages and less red tape. India capitalized on strong chemistry, generic drug development, and a services sector, while China invested heavily in biopharma infrastructure, attracting more than $1 billion in investment from global giants and hundreds of new startups.
“Both countries have a role to play in the global R&D footprint, and both countries have a role to play in the global commercial footprint as well. Obviously China is still the much larger commercial opportunity and will be, at least for the foreseeable future,” Simon Goodall, partner and managing director with The Boston Consulting Group (BCG), told GEN.
With annual growth averaging 22%, more than triple the 6.2% global average, China’s drug market rocketed from $33 billion in 2007 to $126 billion in the first seven months of 2011. The 12th Five-Year Plan (2011–15) commits 2 trillion yuan ($317.75 billion) to biopharma, one of seven strategic sectors expected to comprise a combined 8% of GDP. China hopes to expand its biopharma workforce from 250,000 to 1 million jobs by 2015.
Both China and India have emerged in recent years as offshoring havens. China has invested heavily in infrastructure, while India has capitalized on a strong services sector. [zennie/iStockphoto.com]
Major Chinese Program
In January, China issued its National Program on Bioscience Technology Development 2010–2020. Goals include producing a “biotech talent pyramid” with three to five “worldclass” scientists in major diseases and biofuel; 30 to 50 innovators in teams focused on genomics, stem cells, cloned animals, and neuroscience; plus 300 to 500 “leaders”, 3,000 to 5,000 senior managers, 30,000 to 50,000 “specialists,” and 300,000 bioindustry “experts” in biomedicine, bio-agriculture, biomanufacturing, and bioenvironment.
Many will fill positions at the new big pharma R&D centers, where they will be paid an average 60% to 70% of U.S. salaries. However, the wage gap is shrinking due to Chinese inflation and the demand by companies exceeds the supply of researchers, according to Jim J. Zhang, Ph.D., president and managing director of JZMed, which provides outsourcing consulting to Western biopharmas expanding into China.
Officials will also need to upgrade the nation’s government R&D infrastructure over the next few years. Between 2006 and 2010, according to BCG, China’s biopharma industry nearly tripled its R&D investment to $3.5 billion.
“I think there is a recognition that the SFDA [State Food and Drug Administration] oftentimes can be slower than companies in terms of their review times and the processes to begin clinical trials, particularly those related to first in human studies in China,” said Michael Choy, a principal in BCG’s Shanghai office.
“While the 12th Five-Year Plan can certainly provide a boost to programs and companies, it’s also, I think, a signal of some of the things that are to come that will also be very important to unlocking innovation in China.
China’s biopharma boom has spawned efforts by industry groups in California and Massachusetts to engage—and, they hope, grow—with the world’s most populous nation. In April, BIOCOM, the industry group for San Diego and southern California, completed a trade mission to China aimed, in part, at persuading local biopharmas to expand into the San Diego region.
Biopharma in San Diego County employed 41,937 in 1,705 establishments last year. University of California, San Diego and numerous research institutes anchor a region where Merck and Johnson & Johnson have created incubators to nurture startups alongside home-grown giants that include Gen-Probe, which is being acquired by Hologic, and Illumina. San Diego biopharmas won $835 million from NIH (FY ’11) and $498 million in venture capital (2011).
Joseph Panetta, BIOCOM’s president and CEO, said his seventh trip to China in two and a half years left him most impressed with the advancement of Chinese biopharma companies toward innovation.
“Two and a half years ago, you really wouldn’t see anything in the way of a startup that you would recognize as being similar to a biotech company over here,” he explained. “This year, we visited a couple.”
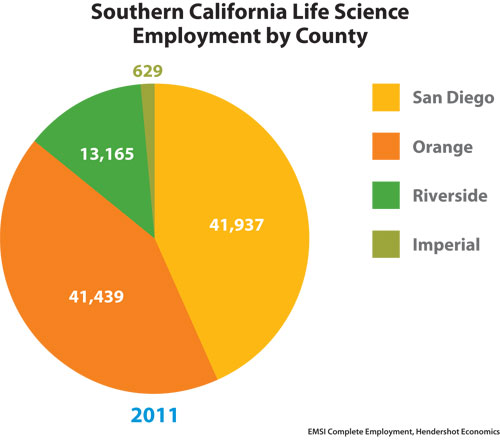
Southern California Life Science Employment by County
Funding Biotech in China
Already the world’s third-largest pharmaceutical market, China is projected to overtake Japan for second by 2015 (IMS). Behind that growth is greater access to private capital secured from three main sources—big biopharma, global financial giants, and, increasingly, venture capital from Chinese investors who made fortunes outside biopharma, and Chinese corporate venture funds, Dr. Zhang said.
“These people have money, so they either form small teams of investment groups, like hedge funds in the U.S., or more formal venture capital investment companies. Both have supported emerging Chinese biotech companies conducting R&D,” he noted.
Dr. Zhang cited the $50 million corporate venture fund of WuXi PharmaTech. China’s largest contract research organization invested $4.3 million as of December 31, 2011, in an undisclosed number of companies, to commercialize their new technologies.
“The more pressing issue for these companies is how and where to invest and whom to partner with,” said David Jiang, Ph.D., managing director of BIOCOM China Consulting.
“Many of them are successful in China now, but to succeed in the next phase of growth, they need to innovate or bring novel products to China. Due to a lack of understanding of the Western culture and products, Chinese companies are apprehensive in making big bets.”
Also engaging with China is the San Francisco Bay Area. In March, BayBio, signed a memorandum of understanding with China Medical City (CMC) in Taizhou to promote collaboration. CMC, founded in 2006, houses more than 200 companies within its initial planned 30 square kilometers (about 11.6 square miles), and is reportedly China’s only science-tech park to receive national government funding plus subsidies from the provincial government of Jiangsu.
“We are seeing some companies here that are looking overseas for services, e.g., contract research, contract manufacturing. There has been some outsourcing to both China and India,” Gail Maderis, BayBio’s president and CEO, explained.
“Small companies are looking to China as a major market so we see them explore Chinese distribution and commercialization opportunities very early on. And rather than just license out global rights, we’re seeing companies look specifically for Chinese or other Asian partners.”
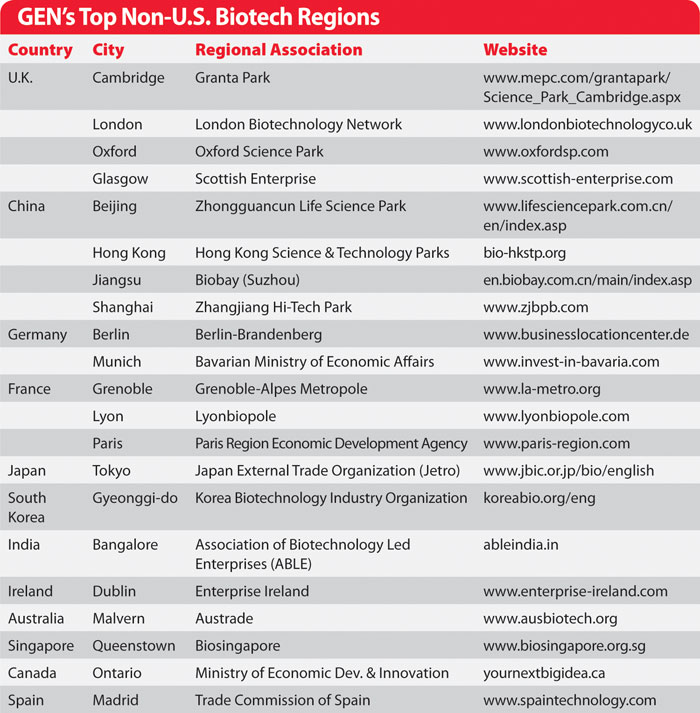
GEN’s Top Non-U.S. Biotech Regions
Largest U.S. Biotech Regions
The Bay Area is the nation’s largest region for biopharma jobs (51,255), businesses (1,377), VC investment ($1.231 billion last year), and NIH grants ($1.368 billion in FY 2011). The region also leads in lab space (20 million square feet, according to Aegis Realty).
The San Francisco and San Diego regions comprise most of California’s life-sci muscle. The Golden State has 2,323 companies generating about $115.4 billion in revenue (2010), a total 267,271 jobs, $1.92 billion in VC last year, and $3.535 billion in NIH grants (FY 2011).
Massachusetts universities, research hospitals, and research institutes won $2.507 billion in NIH grants (FY ’11), while biopharma companies have drawn $1.086 billion in VC funds (2011). Some 3.5 million plus square feet of lab space totaling more than $2 billion is being built by companies, research institutes, and developers, including Alexandria Real Estate Equities, Biogen Idec, Broad Institute, Novartis, Pfizer, and Vertex Pharmaceuticals.
Massachusetts’ 26,807 R&D jobs accounted for more than half (55%) of the Bay State’s total 48,657 biopharma jobs in 2010. Another 9,514 (20%) of Massachusetts’ biopharma jobs are in manufacturing. In recent years, Bristol-Myers Squibb (BMS) completed a $750 million bulk biologics manufacturing site in Devens, while Sanofi’s Genzyme subsidiary completed a new $175 million plant in Framingham, and Shire Human Genetic Therapies completed a $200 million plant in Lexington.
“As companies that are developing new technologies grow, they’ll choose not just to locate their R&D facilities here but their biomanufacturing facilities as well,” predicts Massachusetts Life Sciences Center (MLSC) spokesman Angus McQuilken.
One key reason why is grants and other incentives from quasi-public MLSC, which implements the 10-year, $1 billion Massachusetts Life Sciences Act enacted in 2008 by Governor Deval Patrick. Even before MLSC, state and local governments showered companies with tax breaks like the $60 million BMS won in Devens.
Patrick led a trade mission to China in 2007. This year, for the first time, Massachusetts promoted its diagnostics companies at the China Medical Equipment Fair in Shenzen, while MLSC’s president and CEO, Susan Windham-Bannister, Ph.D., addressed academic-industry collaboration at the International China Pharmaceutical R&D Summit in April in Shanghai.
“We have a growing level of interaction in China. Not as much yet in India, although that’s certainly an area of interest to us,” McQuilken said.
GEN’s Top 15 U.S. Biotechnology Clusters
India’s Bioefforts
India’s nearly 400-company biopharma industry last year enjoyed its best year in the last five with revenues of about $3.2 billion, 21.5% above 2009–10, according to the 9th annual BioSpectrum-Association of Biotechnology Led Enterprises survey. Domestic drugmakers comprised nearly 62% of that due to rising biologics sales, followed by service providers and agri-bio companies.
India’s biggest cities enjoy top-flight clinical infrastructure, but less of it exists in the nation’s smaller cities and rural areas—one of several challenges India must address to stop a decline in clinical trials. Almost 40% of Asian clinical trials occur in China, and 20% in India, a near-reversal of their percentages six years ago, said BCG’s Goodall.
Indian Prime Minister Manmohan Singh has promised to more than double R&D spending to $8 billion or 2% of GDP by the end of India’s 12th Five Year Plan (2012–17). India needs more if it hopes to stay competitive with China.
Rahul Guha, a principal in BCG’s Mumbai office, emphasized that those needs include coordination of its far-flung biopharma efforts and alignment with government goals; reduced regulatory uncertainty; and more investment in drug discovery and clinical research and training as private research investment declines.
During 2010, India recorded just $600 million in “healthcare” deals, including biopharma and other segments. That year, the government launched a $2.2 billion venture fund for drug discovery and research infrastructure development projects.
“VC in biopharma innovation in India would be very, very small. I think it’s close to not there,” Guha said.
India also trails China in attracting back home returnees educated in the West. While returnee programs have drawn hundreds back to India, Guha said, the number accepted under China’s “Thousand Talents” program during its first three years was more than 1,500 (from 6,200 applicants as of Nov. 30, 2011), according to state-run China Internet Information Center.
Looking ahead, India must address its needs for workforce development and increased government spending on research, as China’s ability to succeed at both have aided its rapid rise in biopharma.
The same needs can be said of the U.S., where political bickering has prevented solutions to a decade of flat NIH budgets and a shortage of skilled biopharma workers, while the patent cliff has panicked big biopharma into shifting R&D spending from in-house research to collaborations with biotech partners.
The longer the U.S. and India take to address these issues, the faster China will continue to climb toward global life-sci leadership during this decade.