March 1, 2013 (Vol. 33, No. 5)
Custom Kits for Surgical DNA Alterations Are Now Available
Targeted mutagenesis—that is, altering the genome at a specific site—is a powerful tool for biotechnology. These alterations, whether in cells or in animals, have myriad applications. For example, they can be used to create models of disease by changing a wild-type locus to a mutant one or visa versa, or for developing systems in which to test pharmaceutical products, perhaps by altering an animal gene with its human homolog that is the target of a specific drug.
Most interestingly, it is a method by which very exact gene therapy can be accomplished, allowing surgical-like repair of a defective gene and thus curing disease.
The targeted mutagenesis process has been greatly accelerated in the last few years by the development of synthetic, site-specific double-stranded DNA nucleases that permit the cleavage of DNA at exact locations in the genome, occasionally resulting in small insertions or deletions at the cleavage site. Before this development, mutations could only be introduced by the use of homologous recombination (HR).
In the case of HR, a piece of DNA containing the specific alteration of the genome, flanked by several thousand bases of genomic DNA on either side, is introduced into the cell. At low frequency, this DNA inserts into the genome. Usually this insertion is random, but some small percentage of these insertion events occur at the desired site, replacing the original sequence with the desired alteration.
The synthetic, site-specific double-stranded nucleases, primarily the TALENs and ZFNs (TAL effector nucleases and zinc finger nucleases), can significantly increase the frequency of targeted mutagenesis via HR. These enzymes can be designed to bind almost any unique sequence in the genome, where they will introduce double-strand breaks in the DNA.
While both ZFNs and TALENs are effective at cutting, TALEN targeting follows relatively simple design parameters, is not context dependent, and can generally target more genomic addresses than ZFNs. One of the natural mechanisms for repairing such breaks, universal to all eukaryotic cells, utilizes the intact DNA in the homologous chromosome or sister chromatid as a template for repair, such that the break is repaired without a mutation.
However, if the DNA break is introduced in the presence of a targeting vector, then this altered DNA sequence is utilized as the repair template, with the result that the exogenous change is now introduced into the DNA. Thus, the combination of a site-specific nuclease and a targeting vector will dramatically increase the efficiency with which the desired change is introduced (Figure 1).
Although a site-specific nuclease may increase the frequency of HR at a desired site by several orders of magnitude, in many cases efficiencies are still not extremely high and one must have some way to select for the desired mutation. Typically, this is done by using a selectable marker—that is, a gene encoding a protein that renders the cell identifiable (e.g., it is now resistant to a drug or is fluorescent, such that drug treatment or cell sorting can be used to kill undesired non-altered cells or select and concentrate the desired cells).
This strategy works quite well, but also introduces another complication as the presence of the selectable marker may interfere with the expression of the altered target gene. Thus, an ideal gene-editing system must include some mechanism by which this marker can be excised cleanly from the genome once the desired mutant cells have been selected.
Until recently, the typical solution has been to surround the selectable marker with the target sequences for the Cre recombinase. This enzyme, derived from a bacteriophage, catalyzes recombination between these target sites (known as loxP sites). If the loxP sites are in the same orientation, the intervening selectable marker gene is excised. However, this process is not clean as it leaves behind one of the 34 base pair loxP sites. This remaining “footprint” is not ideal when creating a mutation, especially for human gene therapy or regenerative medicine applications.
Fortunately, a novel solution now exists, utilizing the piggyBac™ DNA transposon.
DNA transposons, sometimes called “jumping genes”, are naturally occurring mobile DNA elements that encode the gene for an enzyme, a transposase, that is flanked by a pair of inverted terminal repeats (ITRs). The transposase recognizes these ITRs and catalyzes the excision of the transposon (and anything encoded between its ITRs) from its resident site and occasionally then inserts it elsewhere within the genome.
While most transposons leave behind some mutation when they are excised, the piggyBac transposon is unique in that its excision restores the sequence of the DNA that existed before the insertion occurred. That is, excision is footprint-free. The transposase gene does not need to be encoded between the ITRs, but rather can be provided in trans and catalyze the footprint-free insertion and excision of any DNA sequence flanked by piggyBac ITRs.
Thus, by flanking selectable markers with ITRs and placing this artificial transposon in a targeting vector, one can then both select for the desired gene modification and then completely remove the selectable marker in a clean and footprint-free manner.
The stage is thus now set for rapid, efficient, and footprint-free gene alterations by combining a site-specific double-stranded nuclease with piggyBac-based selectable markers incorporated into a targeting vector.
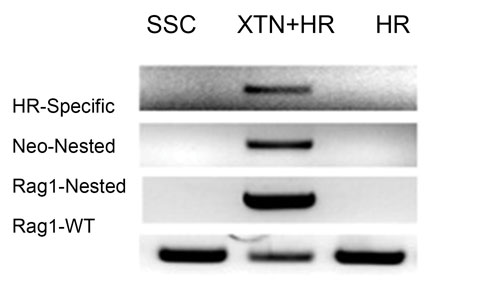
Figure 1. Homologous recombination (HR) in rat spermatogonial stem cells (SSCs) only occurs at high efficiency in the presence of XTNs—in this case, targeting the rat Rag1 gene.
This is the approach offered by Transposagen in its Footprint-Free™ Gene Editing Kits. These combine their XTN™ (xanthamonas TAL nuclease) TALENs, which are built using the high-throughput FLASH™ TALEN assembly system, with custom targeting vectors incorporating the piggyBac DNA Modification System.
Transposagen now offers both hyperactive and excision-only versions of the transposase. Researchers can simply identify the gene being targeted, indicate the alterations desired, and define the desired selectable marker.
In short order they receive an XTN TALEN, a targeting vector that contains the requested gene alteration and a selectable marker flanked by the piggyBac ITRs, and a plasmid encoding a transposase. The customer then co-transfects the target cell line with the XTN TALEN and targeting vector and selects for correctly targeted clones. Once established, these clones can be transfected with the transposase to cleanly excise the selectable marker, leaving behind only the desired change—in other words, Footprint-Free Gene Editing (Figure 2).
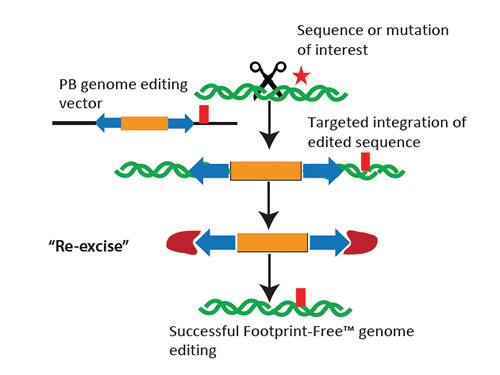
Figure 2. How Footprint-Free gene editing works
Carlisle Landel, Ph.D. ([email protected]), is director, transgenic services, and Ken Miller is senior scientist at Transposagen.



