May 15, 2013 (Vol. 33, No. 10)
New Technology Enables Primary and Secondary Screening of Broad Panels
Luminescence and fluorescence-based assay methods prevail in modern HTS. Although invaluable as technologies, the problem of compound-related assay interferences such as quenching, auto-fluorescence, and light scattering becomes important. The degree of interference experienced may depend on variables such as assay reagents, composition of the chemical library, and reader technology.
Where the amount of interference is unacceptably high, the only option is to delete the data and thus lose all information pertaining to compound activity. False positives and negatives occur when such interferences go undetected. Fluorescence lifetime is increasingly being viewed as an alternative reporter since it largely mitigates such issues.
As a result of significant improvements in assay reagents and the availability of HTS-compatible readers, fluorescence lifetime (FLT) has recently gained new appeal as a screening technology. FLT (also referred to as tau or τ) is the average time between excitation of a fluorophore and its return to ground energy state.
In simple systems, fluorescence intensity decays exponentially, defining FLT as the time in which the fluorescence intensity decays to 1/e (~37%) of that immediately following excitation. FLT provides an attractive reporter modality as it is largely unaffected by auto-fluorescence, light scattering, and inner filter effects providing more robust assays. Furthermore FLT, being an intrinsic parameter of the fluorophore, is independent of probe concentration, which facilitates miniaturization into high-density microplate formats, making FLT a highly attractive and enabling reporting technology for HTS and profiling applications.
There are two approaches currently employed for the measurement of fluorescence lifetimes in microplates: time-correlated single photon counting (TCSPC) and real-time decay curve analysis (RT-DCA). To enable such measurements, the optics in readers comprise a pulsed laser as an excitation source, emission filters, and sensitive photomultipliers tube (PMT) detectors. The approaches differ in the method used to quantify fluorescence emissions in the time domain.
TCSPC involves measuring the time for the first emitted photon to reach the PMT detector following a brief excitation pulse. Repeating this measurement produces a histogram of detected “first photons.” Commonly, TCSPC experiments are set up to record 3,000–10,000 counts in the peak channel resulting in plate read times (384-well) of circa 5–20 minutes. Shorter acquisition times can be utilized for certain applications but may compromise data quality.
In real-time decay curve analysis (RT-DCA), the entire emission curve is recorded after a single excitation pulse, enabling high-throughput time-resolved data to be acquired. The Ameon™ microplate reader (TTP Labtech) contains a digitizer that is capable of detecting thousands of photons per laser pulse enabling plate-read times (1,536-well) of ~2 mins. This makes it possible to implement FLT measurements in studies which were previously challenging, including HTS.
The FLEXYTE Assay Platform
Harnessing the power of FLT requires reagents with appropriate spectral and biochemical properties. The long FLT (17 nanoseconds) of the fluorophore 9-aminoacridine (9AA) is ideally suited for assays, enabling discrimination from interfering species that typically have shorter lifetimes in the 1–5 ns range. By employing 9AA-labeled peptide and protein substrates, the FLEXYTE™ assay platform (Almac Group) has been configured for a number of important drug targets including proteases, protein kinases and phosphatases.
In these assays, the activity of the enzyme is directly reported by a change in the measured FLT, providing a homogeneous, non-radioactive, and antibody-free approach. As an illustration of this platform technology, FLEXYTE assays for two applications are outlined, one in the epigenetics field and the other in ubiquitin drug discovery.
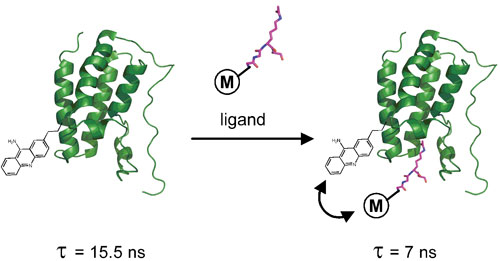
Figure 1A. BRD4(1) FLT assay principle.
In drug discovery, the disruption of protein-protein interactions is increasingly being targeted for therapeutic applications. In epigenetics, proteins termed epi-readers (such as the bromodomain-containing family of proteins) are of particular interest, as they recognize post-translational modifications on histones and play key roles in transcriptional regulation and chromatin remodelling.
The FLEXYTE BRD4(1) protein-ligand binding assay employs the BRD4(1) domain specifically labeled with 9AA, and an acetylated histone H4 peptide incorporating a FLT modulator residue to effect a change in the lifetime of 9AA when the ligand and protein are complexed (Figure 1A). Inhibition of this interaction is simply reported through a change in FLT (Figure 1B).
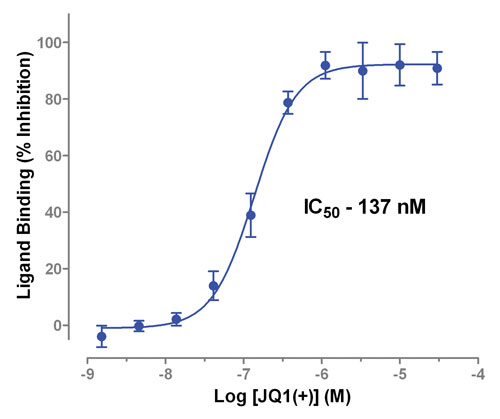
Figure 1B. Inhibition of BRD4(1) binding to histone H4 acetylated peptide by the small molecule inhibitor JQ1(+).
There is a growing interest in targeting the deubiquitylating family of enzymes (DUBs) for drug discovery, given their increasing implication in a variety of different diseases. The FLEXYTE FLT DUB assay platform combines the benefits of FLT reporting technology with “physiologically relevant” isopeptide substrates to provide a robust screening methodology (Figure 2A).
Ubiquitin is generated with the 9AA FLT reporter site-specifically attached to the C-terminus of the protein via a lysine isopeptide bond. A known FLT modulator (M) is incorporated at a selected site within the ubiquitin domain, which serves to modulate the FLT of 9AA in the substrate. Enzymatic cleavage of this scissile isopeptide bond is reported, in real time, through a change in FLT of the system (Figure 2B).
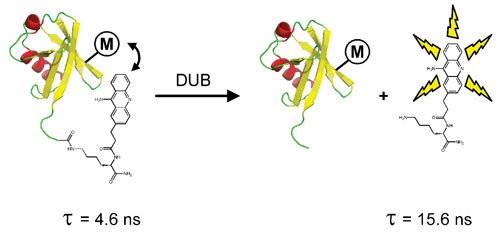
Figure 2A. FLEXYTE isopeptide-DUB assay principle.
Correcting Assay Interferences
Unlike many other fluorescence-based technologies, advanced FLT acquisition and data-analysis capabilities can rapidly identify, discriminate, and eliminate results from interfering fluorescent compounds. The information-rich FLT output allows determination of additional fluorescence characteristics, including amplitude and intensity. These characteristics can be interrogated to distinguish between fluorescence from interfering compounds with similar emission profiles to FLT probes.
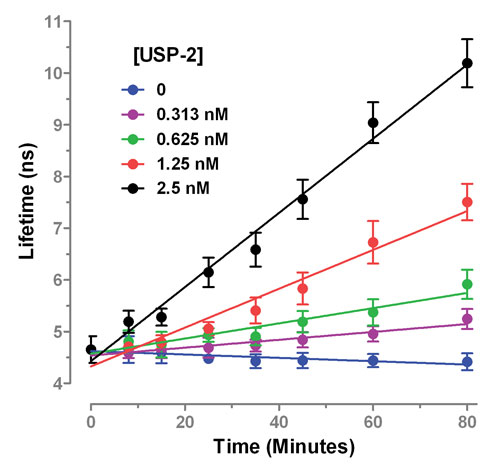
Figure 2B. Activity of the DUB enzyme USP2 is reported in real time through a change in FLT.
The improved data quality from RT-DCA allows re-analysis and correction of the data by applying multi-exponential algorithms to account for the contribution of the interfering fluorescent component (Figure 3). Hence, FLT technology enabled with RT-DCA has the capacity to not only flag compound-related interferences but to also correct for their effects, leading to a reduction in the number of false positives and negatives and an expanded dataset.
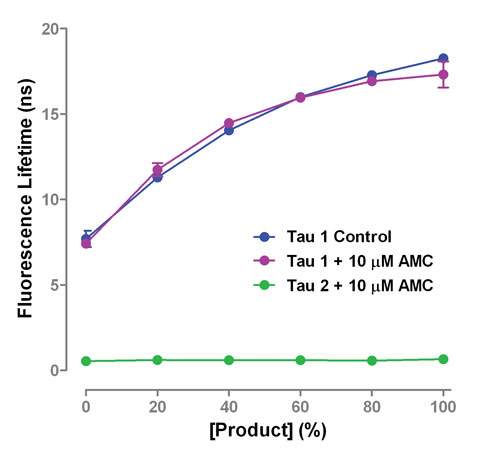
Figure 3. Addition of fluorophore 7-amino-4-methylcoumarin (AMC) to a FLEXYTE caspase-3 assay has a dramatic effect on the fluorescence decay curves. Unlike other fluorescence-based assay techniques, the information-rich RT-DCA output permits analysis and correction of the data.
Conclusion
FLT technologies complement the portfolio of established assay formats by enabling primary and secondary screening for broad panels of some of the most exploited drugable enzyme families. Its flexibility also enables previously intractable targets in the expanding field of epigenetics to be easily addressed. The combination of FLEXYTE assays and Ameon microplate reader provides a revolutionary combination of speed, precision, and data quality for FLT assays that can be readily integrated into HTS workflows.
As such, they deliver robust, cost-effective screening results, enabling better discrimination of “hits” from false positives or negatives, which are so prevalent in intensity-based screens.
Graham Cotton ([email protected]) is senior R&D group leader at Almac Group, and Wayne Bowen ([email protected]) is CSO at TTP Labtech.



