June 1, 2012 (Vol. 32, No. 11)
Josh P. Roberts
Everything in chromatography is a compromise. It’s like a game, trying to come up with the best system of compromises that gives you the optimum platform for the separation process.
“There are lots of ways you can do it,” explains Jack (J.J.) Kirkland, Ph.D., vp for R&D at Advanced Materials Technology (AMT), who will be among those speaking at CASSS’ “HPLC” conference later this month. “What you try to do is balance all the parameters that are involved and try to come up with the best thing you can.”
The theory suggesting that smaller particles in an HPLC column increase the ability to separate components goes back at least to the early 1960s, recalls Dr. Kirkland.
Another critical parameter is that “you want enough surface area so you can load a sample that you need, and particles creating the required surface area to give you the kind of retention that you need.” By the early 1970s, both of these needs were addressed with small—5–7 µm—totally porous particles. Pores wending through the particles increased the surface area.
But the further a molecule has to diffuse into the totally porous shell, the more time it takes. “More time for diffusion means a broader band, and that means less column efficiency,” he notes. This is especially important for larger molecules such as peptides and proteins for which diffusion can be rate-limiting for separation speed.
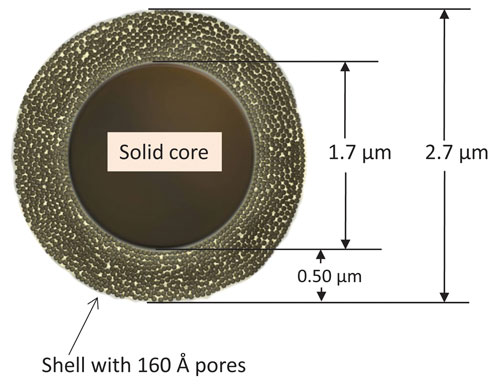
But what if you could make smaller particles—say, 2.7 µm—with pores that don’t go all the way through, so that the diffusion path is much shorter? Such superficially porous particles (SPPs)—which AMT introduced in 2006—allow “you to run faster mobile phases, increase the efficiency of the system, and reduce the separation time,” Dr. Kirkland says. They have an efficiency equivalent to totally porous particles of 1.8 µm.
The reduction of surface area means that the amount of sample that can be loaded onto SPPs is less than what totally porous particles can handle. But Dr. Kirkland notes that because mass spectrometry (MS)—which many people are doing—doesn’t require a lot of sample, this isn’t a big problem.
AMT recently introduced 2.7 µm SPPs with wider pores to insure unhindered access of larger solutes to the porous shell for rapid, efficient separations. Because the pressure required to drive the mobile phase through the column goes up with the square of the particle size, 2.7 µm is about as small as particles can be and still run on conventional HPLC equipment—sub-2 µm particles require expensive, specialized high-pressure (UHPLC or UPLC) equipment with optimum separation speed, he points out.
Particles packed into Advanced Materials Technology’s HALO® columns are manufactured using Fused-Core® particle technology that was developed to deliver fast chromatographic separations.
While such an apparatus is now in wide use, it’s “still beneficial to the people who use the columns day in and day out to operate at lower back pressure,” says William Barber, Ph.D., Agilent Technologies’ R&D manager for liquid chromatography columns. There is less stress on the instrument and the column, therefore extending the interval between maintenance of the instrument and lengthening the effective lifetime of the column.
Agilent has for years offered larger particles (e.g., 3.5 µm and 5 µm) with a 300 Å pore size, designed for biochromatography on standard HPLC columns and recently commercialized a 1.8 µm line of 300 Å pore size columns for use with UHPLC.
About eight years ago Agilent tested the effect of broadening the particle size distribution on the permeability and efficiency of its small molecule (80–120 Å pore size) UHPLC columns. Its manufacturing process generates both spheres (unimers) and also a small percentage of fused particles (dimers), with the vast majority of dimers removed prior to packing the columns to market.
Dr. Barber is currently conducting similar experiments on the 300 Å pore particles. “We systematically add dimers back in known quantities, to more systematically study what our process would look like if we left a certain amount in or varied the amount of dimers that were left in the product.”
The results for the large molecule columns are similar to those seen for the small molecule columns. “We still see the same trend where you can add some of these dimers back and it really doesn’t have any effect on the efficiency up to about 10–20% of the dimers,” Dr. Barber says. “But you do tend to see a consistent change in permeability or back pressure right from the beginning, as soon as you start adding dimer.”
“We haven’t done all the measurements yet,” so the results are still preliminary, Dr. Barber points out. For example, they still want to do van Deemter plots on all the materials to see how the performance versus flow characteristics change with the different mixtures; to test different size molecules to determine whether molecular weight plays a role; and to query different modes of chromatography.

Agilent Technologies’ manufacturing process generates both unimers (shown) and also a small percentage of dimers.
The Tower
An alternative to UHPLC columns are monolithic columns, so-called because they are made from a single piece of porous material.
Because of the manufacturing process, Merck KGaA’s monolithic silica columns are surrounded by polymeric polyether ether ketone (PEEK), and cannot withstand the same kind of back pressures as traditional stainless steel particle packed columns can. But that doesn’t seem to be a problem since they possess a high permeability and are designed to yield fast, high efficiency separations using “conventional low pressure and cheap HPLC systems,” says Karin Cabrera, Ph.D., head of R&D for analytical chromatography at Merck Millipore.
In monolithic columns, it is the size of the through-pores (macropores) that determines permeability, while the mesopore size determines the surface area of the material and thus the separation efficiency. “We can independently design the macropore size and the mesopore size,” extolls Dr. Cabrera.
This is not possible with the classical particle packed column, where the interstitial volume corresponds to the macropore size, she continues. Smaller particles yield a smaller interstitial volume and require a correspondingly higher back pressure to operate. Yet separation efficiency is dependent on the particle size as well.
As there are no frits to clog, “dirty” samples can be injected directly on to monolith columns without the need to filter them. In addition, because the column is a single rigid piece, it has been shown to last much longer than packed columns, which are subject to shifting and cracking over time.
The new Chromolith HR columns, with a 1.1 µm macropore size, can operate at 3.5 mL/min at a back pressure of only 200 bar, with a measured efficiency surpassing that of a 3.0 µm packed particle column. “We started with 2 µm [macropore] and now with the second generation we have 1.1 µm; now of course we can go down further—to 0.9 µm, 0.8 µm , 0.7 µm, 0.5 µm, etc.,” Dr. Cabrera says, predicting that in the next five years “we will reach efficiencies that cannot be obtained any more with smaller particles.”
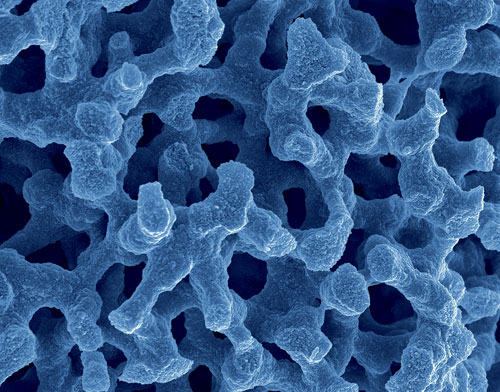
Scanning electron microscope image of a monolithic column: In monolithic columns it is the size of the macropores that determines permeability and the mesopore size determines the surface area of the material and thus the separation efficiency. [Merck]
Pass the Salt
Ion chromatography (IC) columns use an organic sheathing as well, and thus cannot use ultra-high pressure systems—but for different reasons. “There are no metal parts in IC instrumentation,” points out Yury Agroskin, director of R&D at Thermo Scientific. “We cannot use stainless steel pumps because we use a very broad range of pH—from 0 to 14. Stainless steel material can’t withstand this continuous corrosive environment.”
Yet the same rules still apply—the system’s resolving power is inversely proportional to the particle size. Thermo has introduced the new Thermo Scientific Dionex ICS-5000 Reagent-Free™ HPIC™ system with Eluent Generation, a high-pressure capillary IC system.
It uses columns with 4.0 µm particle size and runs at up to 5,000 PSI. While these numbers seem tame (UHPLC systems can push 20,000 PSI through columns packed with 1.7 µm particles), traditionally IC standard pressure is below 3,000 PSI, with 8–12 µm particles.
The high-pressure system is combined with a 0.4 mm ID capillary column to offer an “always-ready” chromatographic system that can run continuously—literally months and months—without human intervention,” Agroskin noted. “The flow rate is extremely small—we’re talking in the range of 10 µL/min.”
This, he points out, simplifies things for the customers, who don’t need to worry about starting up, equilibrating, and calibrating the system each time they need to use it. It also minimizes the consumption of environmentally unfriendly substances.

The Dionex ICS-4000 capillary HPIC™ system delivers high-pressure capability, according to Thermo Scientific, and its integrated design simplifies workflow and increases analytical efficiency and productivity.
Mind the Carbs
Characterizing proteins is an important task of chromatography. And while modern HPLC- and UHPLC-MS techniques have brought us a long way toward solving many of the challenges of working with glycan structures, the heterogeneous nature of glycoproteins continues to cause a bottleneck in the biopharmaceutical industry.
Critical quality attributes of post-translationally added structures may affect the safety and efficacy of the proteins they are found on. Bioactivity, distribution, metabolism, and clearance, for example, may all be dependent on the makeup and placement of sugar trees decorating a protein-based drug.
It’s important to the people in the industry to “make sure they have a consistent process and they get a consistent glycan distribution, not only in terms of those glycan structures that are added to the protein, but the site of that addition as well as the relative amounts or distribution of these,” notes Steven Cohen, Ph.D., RDE life sciences director at Waters. This is especially true for biosimilars, which by definition are produced in a different cell line.
There are three levels at which these structures are typically analyzed, each yielding its own set of information, and all combining to give a fuller characterization of a protein: the intact molecule, which yields no information about where the glycan is attached; glycopeptides; and the glycan itself, released by enzymatic or chemical means, “which allow you to get in-depth information about both the glycan composition and well as distribution among various glycan forms,” Dr. Cohen explains.
He sees the underappreciated in-between stage of glycopeptide analysis—that can show to which peptide the particular glycans are attached—as having greatly benefitted of late by improved separation technologies.
“Although you can get complete analyses in 30 minutes with a UHPLC-type column, those columns allow you to get very good screening information in under 10 minutes. That’s very useful for high-throughput analysis.”