July 1, 2010 (Vol. 30, No. 13)
Vision Technology Is Being Used to Facilitate Liquid Volume Auditing and Precipitate Detection
Knowing what quantity of items you have in stock is a basic requirement for anyone involved in inventory management. Knowing that the quality of the items has not deteriorated as a result of storage is also important. This not only ensures that your end customer always receives a quality product, but it also minimizes waste and thus costs.
While this has been well understood in businesses involved in perishable goods warehousing and distribution, it is now increasingly important in sample-management applications within the pharmaceutical and biobanking sectors.
During the last decade, organizations in these sectors have invested heavily in sample-management solutions to deal with ever increasing numbers of samples, whether they be small molecule compounds or biological samples.
This investment often takes the form of automated storage and retrieval systems. Many millions of samples may need to be stored. Automation, and the associated IT and data tracking infrastructure, is the only realistic solution to providing rapid controlled access to individual samples, many of which will be stored at sub-zero temperatures.
In most cases, samples are stored in solubilized form in microtubes. Knowing how much sample is in the microtube and an indication of the quality of that sample is thus key. After all, what could be worse than keeping the sample in storage only to find that when you need to access it, there isn’t the amount that you expected. In the worst-case scenario, it could actually be empty if someone has used it all. Additionally, if the sample has degraded during its time in storage then this will likely impact on any subsequent analytical processes to be performed on that sample.
In the drug discovery process, compound managers typically deliver samples in SBS microplate format to downstream screening groups. They need to have confidence in the quality of their delivered product (i.e., the plate contents). If there are empty wells in the microplate, or perhaps wells with samples at the wrong concentration, this can have a potentially significant effect on the results from the screening process or other downstream analyses being performed. A secondary effect of this is potentially significant levels of waste and cost.
Data gathered by RTS Life Science suggests that in some organizations as many as 5% of plate wells may be erroneously empty and 3–5% of wells may have samples at the wrong concentration. Knowing the volume of sample in the source microtube used to create the plate, and whether there is any precipitate, is key to avoiding screening empty wells or samples at the wrong concentration.
What is required, therefore, is a rapid and accurate method by which liquid samples can be audited so as to determine if there is any sample in the tube, what the volume of sample is in the tube, and if any of the sample has precipitated out of solution.
Existing Approaches
There are a number of approaches currently used for determining the volume of liquid sample in a tube. These include gravimetric, ultrasonic, and other liquid-level sensing methods (e.g., capacitive probe). However, each of these approaches has certain disadvantages, as summarized in the Table.
Furthermore, these methods cannot determine if any sample has precipitated out of solution. While a manual visual check can help spot precipitates, this is a very onerous task if large numbers of samples need to be audited.
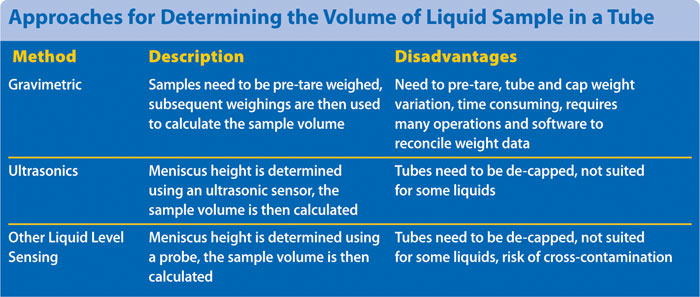
Approaches for determining the volume of liquid sample in a tube
Making Sample Quality Visible
A new solution to this challenge uses high-resolution vision technology to audit both volume and precipitate in racks containing up to 96 tubes. An advantage of this approach is that auditing can take place without de-capping of the tube, thus minimizing any exposure of the sample and helping reduce potential degradation or cross-contamination.
A high-speed vision system incorporated into a benchtop instrument is used to image multiple sample tubes simultaneously—up to a full column of eight tubes in a standard SBS footprint rack. The captured image is analyzed, and the liquid meniscus detected for each tube being audited.
Advanced image processing routines take into account variations between different liquid types, for example DMSO or aqueous solutions.
Once the position of the meniscus has been determined, the volume of liquid is calculated, taking into account the tube type. Further algorithms are used to detect the presence of a cap and any precipitate in the base of the tube.
Figure 1 illustrates a column of eight tubes following image capture, with various regions of interest depicted, with detection of caps, meniscus, and precipitate (in two of the tubes).
Benefits of this approach include: speed, less than two minutes to audit a 96 tube rack; accurate, volume measurement better than +/-10 µL; precipitate and cap detection; no need for tare-weighing of tubes; noncontact and operates with capped tubes.
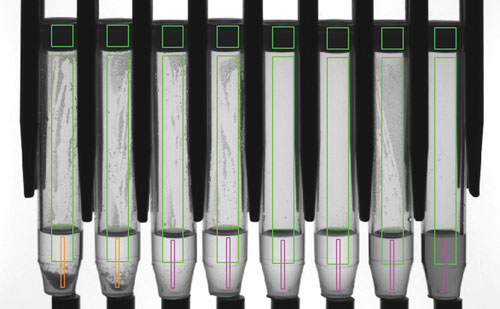
Figure 1. Cap detection, volume, and precipitate level detection as measured by the Tube Auditor
The RTS Tube Auditor™ (Figure 2) can be used manually, with racks of tubes presented by the user, or integrated into a fully automated system, with racks of tubes being loaded/unloaded by a robot. This allows end users to audit small or large numbers of tubes according to the needs of their organization.
By integrating the instrument into an automated system, typically where the liquid sample is aspirated from the source tube and dispensed into a plate, it is possible to perform auditing following selected, or indeed every, access to the sample. Users can determine the frequency at which they audit samples based on their own quality control processes.
Although originally intended to facilitate in-process auditing, this technology is also finding applications at an earlier stage in the drug discovery process. Having an accurate record of the volume prior to putting the sample into storage is clearly important.
The Tube Auditor can be used to measure volume at the time of initial sample dissolution (and also give an indication of how well it has been solubilized). During early trials the technology reliably identified samples with lower volumes than had been anticipated. Upon investigation, this was found to be a result of blocked tips in the liquid handler used to perform the solubilization.

Figure 2. The Tube Auditor is a benchtop or integrated unit for volume measurement and precipitate detection in SBS format microtubes.
Other Applications
In addition to compound management and HTS applications, this technology has been found to exhibit good performance when auditing DNA (in buffer solution) as well as other biological fluids. Other applications where the technology is proving of interest include biobanking, sample QC and empty tube detection, sample dissolution and solubility quality checks, and cap detection (to avoid liquid-handling tip damage).
As users become aware of the possibilities that vision technology brings, it is highly likely that new applications will arise. A recent example of this is the detection of the presence of mixing beads in sample tubes.
Conclusion
By applying vision technology, users now have access to a high-accuracy methodology for auditing liquid sample volumes and detecting precipitates that takes significantly less time than existing methods.
Onerous manual visual checks, or slow automated processes, can be eliminated, allowing regular monitoring of sample tubes. This increases confidence in the quality of the sample being stored and reduces the impact of variable quality on downstream processes such as screening and analysis.
Chris Walsh ([email protected]) is sample management product manager at RTS Life Science.



