May 15, 2013 (Vol. 33, No. 10)
Mono- and Polyclonal Antibody Development Against Challenging Antigens Just Got Easier
Today’s biopharmaceutical industry faces significant challenges identifying antibody reagents useful in the development of difficult therapeutic and/or diagnostic applications. Target-specific antibodies, irrespective of clonality, are traditionally developed and produced in wild-type animals upon immunization with a specific antigen. However, the complexity and/or lack of immunogenicity of certain antigens can prevent the successful development of suitable, assay-specific, diagnostic, or therapeutic antibodies.
Researchers who were unsuccessful in developing antibodies in wild-type animals could attempt the immunization of “knock-out” animals with the hope of stimulating an antibody-mediated immune response against conserved antigens to eliminate or minimize immune tolerance; however, should such undertakings fall short as well, no further alternatives have been offered until now.
The inherent complexity of native or recombinant protein or peptide-based antigens, including high homology, conserved functional domains, and/or the cyclic nature, present great difficulty in triggering an immune response in both wild-type and knock-out animals and often confers toxicity together with animal suffering without achieving the desired outcome. Antigen presentation and immunoglobulin preservation are crucial factors to develop highly specific and/or functional antibodies against difficult targets.
A key technology to address and overcome the aforementioned scenario has been developed by ImmunoGenes, in which Balb/c mice and New Zealand white rabbits have been genetically engineered to overexpress the neonatal Fc receptor (FcRn) specific for immunoglobulin gamma (IgG). The role of FcRn in antigen presentation has recently been elucidated and explains the robust augmentation of the immune response on both quantitative and qualitative levels. This renders ImmunoGenes’ FcRn transgenic animals ideally suited for monoclonal and also polyclonal antibody development against a variety of difficult antigens.
Transgenic mice antibody titers exceed those observed in immunized wild-type counterparts by three- to fivefold. In addition, spleens harvested from transgenic mice were found to be up to two times larger, containing higher counts of splenocytes or B cells as well as other cell types to that observed in immunized wild-type controls and most importantly antigen-specific plasma cells (Figure 1).
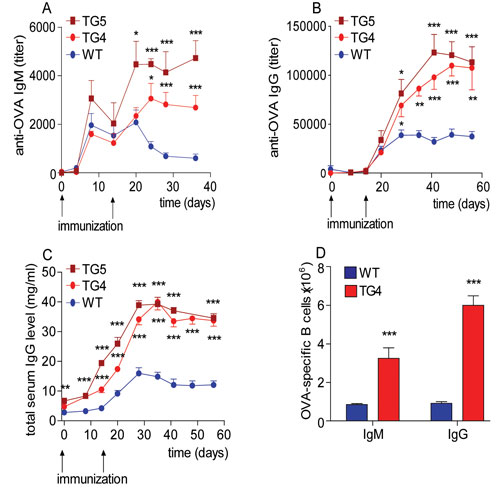
Figure 1. FcRn overexpression results in a robust augmentation of the immune response in transgenic (TG) mice carrying four and five copies of the FcRn respectively, in comparison to their wild-type controls when immunized with ovalbumin (OVA). (A) OVA-specific IgM and (B) OVA-specific IgG titers were nearly tripled during the secondary immune response in TG mice compared to wild-type animals. (C) Transgenic mice produced significantly higher amounts of total IgG compared to wild-type mice. (D) ELISPOT assays were performed to test for the presence of OVA-specific B cells. [Figure reproduced from Cervenak et al. 2011. J Immunol. with permission]
In combination, these findings present major advantages during the development of hybridomas for the production of monoclonal antibodies against weakly immunogenic antigens, as was proven by generating high levels of specific antibodies against a highly conserved but not immunodominant influenza epitope in FcRn transgenic mice and not in their wild-type counterparts (Figure 2).
Another major challenge in the development of antibodies against highly homologous and/or conserved antigens is presented by the host’s immune tolerance. A robust humoral immune response in FcRn transgenic mice demonstrated to overcome the animal’s immune tolerance as antibody titers generated against a GPCR-related mouse cannabinoid receptor type 1 (CB-1)-specific peptide, identical to its human ortholog, outweighed the antibody-mediated immune response in wild-type animals immunized with the same.
A broken immune tolerance together with an expanded repertoire of primed and antigen-specific memory B cells, in turn, facilitated the development of ten CB-1-specific hybridomas from FcRn transgenic mice in this delicate case, while hybridoma generation using splenocytes from immunized wild-type mice yielded none. Based on immunocytochemical analyses, some of these anti-CB1-specific monoclonal antibodies are capable of detecting native CB1R with high affinity in the nanomolar range (Figure 3).
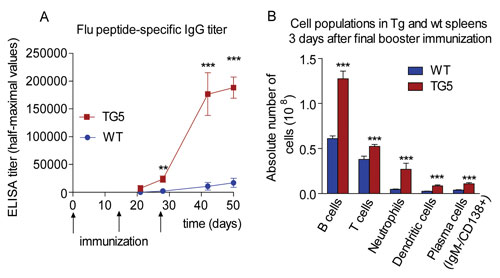
Figure 2. Immunization with HA2-KLH elicits a potent antipeptide immune response in FcRn transgenic mice. (A) HA2-specific IgG titers show a substantial increase in TG mice in comparison to the negligible IgG titers of wild-type mice. (B) Absolute number of B cells, T cells, neutrophils, dendritic cells, and plasma cells were significantly higher in spleens from TG animals as measured by FACS analysis. [Figure reproduced from Vegh et al. 2011. mAbs with permission]
Benefits
Similarly, ImmunoGenes’ FcRn transgenic rabbits have shown an identical robust antibody-mediated immune response against a variety of difficult antigens to that observed in transgenic mice (Figure 1), making them ideally suited for the production of high-quality polyclonal antibody in shorter time periods if desired. Furthermore, immunized FcRn transgenic rabbits can also be used for the development of rabbit monoclonal antibodies, an increasingly sought-after commodity in the biopharmaceutical industry.
FcRn transgenic rabbits are ideally suited for the development and production of anti-host cell protein lysate (HCP) polyclonal antibodies capable of recognizing weakly and highly immunogenic components of the HCP mixture—generally classified as a mixture of difficult antigens, with greater than 90% coverage. In turn, the developed reagent is ideal for equivalent detection of low and high molecular proteins and can be incorporated into clearance studies of new drugs developed and manufactured by the pharmaceutical industry in a variety of different expression systems.
FcRn has been identified in mediating maternal IgG transport, regulating IgG metabolism, significantly preventing IgG catabolism in a concentration-dependent manner, and playing a crucial role in the antigen-IgG immune complex presentation. Overexpression of this receptor in transgenic animals therefore aids in the increased rescue of antigen-specific IgG molecules and improves antigen presentation resulting in greatly augmented antibody-mediated immune responses against antigens that cannot be compared to that potentially induced in wild-type or knock-out animals over the course of an immunization regimen.
The implications of such are that, presently, this alternative technology is accessible to the greater biopharmaceutical and research community for the development of monoclonal or polyclonal antibodies against weakly immunogenic and/or highly homologous antigens when traditional antibody development approaches have not succeeded, making it extremely appealing for the development of diagnostic and therapeutic antibodies incorporated into tomorrow’s disease-specific assays and treatment programs.
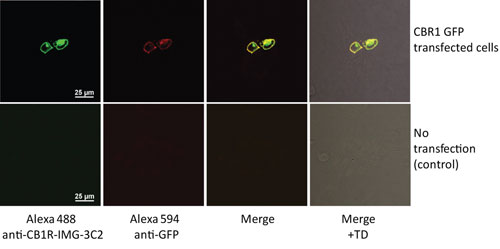
Figure 3. Breaking immunotolerance: FcRn TG mouse anti-CB1 monoclonal antibody IMG-3C2 reacts to a highly conserved C-terminal 31-mer oligopeptide of the mouse cannabinoid receptor type 1 (CB1R) that is identical to its human ortholog. [Figure reproduced with permission from István Katona, Ph.D., Institute of Experimental Medicine, Hungarian Academy of Sciences]
Thomas O. Kohl, Ph.D. ([email protected]), is director, customized antibody solutions at ImmunoGenes.



