November 1, 2011 (Vol. 31, No. 19)
Josh P. Roberts
Improvements in Assessment and Manipulation Help Scientists Get to the Bottom of Previously Intractable Problems
It’s hard to imagine a biomedical field that’s not concerned with cell signaling. From immunologists studying GPCRs and neuroscientists investigating amyloid plaques, to cardiologists telling their patients to avoid caffeine, knowing what’s connected to what and which kinases are phosphorylating which others is all part of the daily grind.
At Cambridge Healthtech’s “Discovery on Target” conference, held earlier this month, pathways leading to and coming from PI3 kinase seemed to be in the forefront of many researchers’ minds. Yet, generating tools to assess and manipulate the status of all sorts of pathways—from those involved in cancer to those responsible for addictive behavior—was of interest no matter what your favorite signaling molecule.
It’s notoriously difficult to generate functional antibodies against complex cell-surface molecules. Antibodies are typically generated against “some kind of artificially expressed and folded GPCRs that don’t always have their native conformation,” remarked Sergej Kiprijanov, Ph.D., vp of research and preclinical development at Affitech.
Affitech wanted to antagonize GPCRs involved in cancer progression and inflammation. Using its cell-based antibody selection (CBAS) technology it screened its massive phage-display human antibody library against receptors (such as the CCR4 and CXCR4 chemokine receptors) expressed on the cell surface in their natural conformation, and identified several candidates able to impact the ability of these receptors to signal.
In the most advanced of six anti-GPCR programs, for example, an anti-CCR4 antibody was found that can deliver a strong response against CCR4+ tumors in xenografts. At the same time, the antibody is able to target CCR4+ regulatory T cells that can inhibit a robust imm?une response against the tumor.
Because of redundancy in the system, one chemokine receptor may have several natural ligands. So, unlike targeting the chemokines themselves, “by blocking the receptor with the same compound you can abolish the signal provided by three or four different chemokines,” explained Dr. Kiprijanov.
SHIP of PIP
Stephen Ward, Ph.D., works further inside immune cells, on signaling pathways involving PI3K. But rather than trying to directly inhibit the nearly ubiquitous kinase itself—risking substantial off-target effects—the University of Bath professor of pharmacy and pharmacology instead tries to focus efforts on a more targeted target.
Expression of the SHIP-1 is primarily restricted to cells of hematopoietic lineage. The phosphatase is responsible for degrading PIP3—the primary product of PI3K—to PIP2, essentially undoing what the kinase has done.
Dr. Ward’s group initially used short hairpin RNAs to silence expression of SHIP-1 in human T lymphocytes and was surprised to find no effect on directional cell migration and an impairment of basal cell mobility. They postulated that perhaps some of the molecule’s actions were the result of a scaffolding function distinct from domain-mediated effects. SHIP-1 encodes multiple structural domains that are known to interact with and recruit other molecules through protein-protein interaction and cellular relocalization.
A series of experiments using small molecules—activators and inhibitors of SHIP-1—revealed that the two approaches “don’t exactly phenocopy each other or the effect of SHIP-1 knock-down,” Dr. Ward noted. “One conclusion, therefore, is that the noncatalytic domain-mediated scaffolding functions may be avoided/missed by small molecule approaches.”
But it may not even be that simple. Some small molecules actually inhibit both basal and activated migration.
Like PIP3, PIP2 also interacts with pleckstrin homology (PH) domains within effector proteins, so altering SHIP-1 activity—whether by a shotgun molecular approach or a more targeted pharmaceutical one—may shift the balance between PIP3 and its degradation product. “And that is critical in determining overall motile response of T cells. The functional impact of disrupting this balance can be difficult to predict given the number of downstream effector molecules.”
Two for One
In tumors in which PI3K is implicated, another kinase, mTOR, is often found to be dysregulated as well. Interestingly, mTOR has never been found to be mutated, but it tends to be dysregulated by things other than mutations—such as a variety of upstream pathways that feed into it.
“These proteins don’t act in isolation,” explained David Matthews, Ph.D., vp for drug discovery and exploratory development at Pathway Therapeutics. “They form a complex signaling network with a lot of feedback loops and interactions between them and between various other cellular components.”
If mTOR is targeted on its own (for example, by a rapamycin analog), these feedback loops will actually serve to activate PI3K. Both proteins, Dr. Matthews said, really need to be targeted to have a robust effect on tumor cell signaling, and Pathway has developed just such a molecule. PWT33597 is an inhibitor of mTOR and PI3Kα, the isoform most frequently dysregulated in tumor cells. PWT33597 is very selective—“it doesn’t hit any other kinases that we can tell.”
The compound generates a balanced inhibition, leading to cell death, in cell-based assays, “and also nice tumor growth inhibition in xenograft models. And that’s the data that has led us to move this molecule forward into the clinic.”
PWT33597 is currently in a Phase I clinical study. In addition to demonstrating safety and tolerability, the company is also looking for pharmacodynamic markers of target inhibition. “To that end we put in place a panel of assays that lets us do this, monitoring PI3K and mTOR signaling in tumors, in blood, and in hair.”
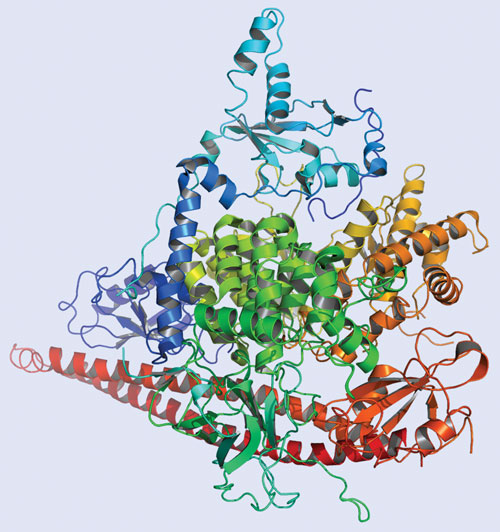
X-ray crystal structure of PI3K alpha, a kinase that is hyperactivated in many tumor types. (Figure generated using the PyMOL Molecular Graphics System.) [Pathway Therapeutics]
Survival vs. Longevity
Perhaps the best characterized downstream effector of PI3K is AKT/PKB (protein kinase B), which interacts with PIP3 through its PH domain. AKT is known to foster cell growth—a good thing when you want to protect cells against things like neurodegenerative disorders, but “obviously during cancer it’s a target that you’d want to limit,” pointed out Kenneth Maiese, M.D., chair of the department of neurology and neurosciences at the University of Medicine and Dentistry of New Jersey.
Dr. Maiese is interested in finding treatment strategies that balance the sometimes competing goals of increasing cellular protection in acute situations with preserving cell longevity. To that end, his lab looks at novel pathways that include transcription factors such as wingless, forkhead, and the sirtuins, and the role they may play in illnesses like Alzheimer disease, stroke, diabetes, and cancer.
Take, for example, the cytokine and growth factor erythropoietin (EPO), which is FDA approved to treat anemia. EPO stimulates AKT, and Dr. Maiese has shown that many of the sub-pathways from EPO lead to wingless and forkhead.
“What we’re trying to do, in a nutshell, is to either design new drugs or, using current drugs like EPO, to use better compounds or derivatives of these compounds that may increase intended efficacy (such as the treatment of anemia) but limit any complications (such as the growth of cancer).”
The pathways Dr. Maiese works on are very complex. Although they traditionally may have been thought to only do one thing, it may surprise people to learn of newly discovered, more novel, connections, he said. “It’s very important to understand how these pathways may function in certain environments, whether they affect acute cell survival, and whether they also affect long-term cell longevity.”
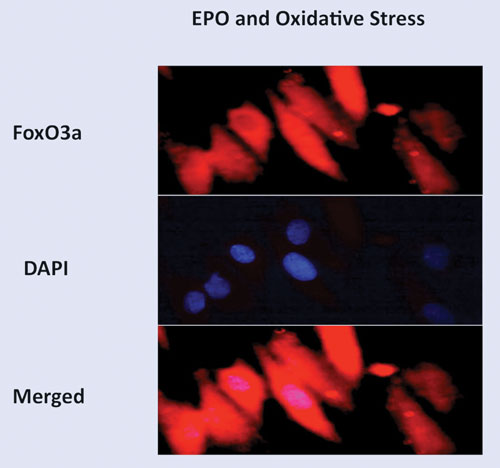
Erythropoietin controls FoxO3a subcellular translocation by maintaining FoxO3a (red in color) in the cytoplasm of vascular cells during oxidative stress and preventing the nuclear transcription of “pro-apoptotic” FoxO3a pathways. The nuclei of cells are identified by DAPI staining (blue in color). Merged imagees illustrate the primary absence of FoxO3a in the nuclus of cells.
Kill the Chaperone
Many cancers are the result of constitutively active JAK-STAT signaling, and attempts to control that pathway are proceeding apace. Incyte’s JAK inhibitor, ruxolitinib, is under fast-track review by the FDA, and other compounds are currently in clinical trials. These ATP-competitive inhibitors target the protein’s kinase activity.
“But, oftentimes, these kinases will mutate and no longer be responsive to their inhibitor,” said David Proia, Ph.D., senior scientist at Synta Pharmaceuticals.
The Boston-based company uses a different strategy to target JAK. Ganetespib is an inhibitor of Hsp90, a chaperone protein essential for the folding of hundreds of client proteins including JAK2. By inhibiting the activity of Hsp90, JAK2 is unable to adapt its active conformation and is subsequently targeted for degradation by the proteasome.
“Regardless of the mutations that develop in a client kinase like JAK they still require Hsp90 for their maturation, and so they’re still candidates of Hsp90 inhibitors,” Dr. Proia pointed out.
Leukemic cell lines driven by JAK2 mutations are highly sensitive to ganetespib, due in part to the degradation of JAK2 and subsequent inhibition of its substrates, STAT3 and STAT5, transcription factors linked to tumor cell survival, proliferation, and metastasis.
Yet, it is its effect on Hsp90’s other clients that intrigues Dr. Proia so much about ganetespib—most specifically the “overwhelming effect that Hsp90 inhibitors have on cell division and DNA replication. It’s the coordinated insult on kinases like JAK, AKT, as well as cyclins like CDC2 and other checkpoint proteins: the culmination of inhibition of those targets I think is what really gives such great potency to ganetespib.”
Despite this, ganetespib doesn’t seem to have the toxicity issues that earlier-generation Hsp90 inhibitors had. Over 400 cancer patients have been treated in the clinic so far—ganetespib is currently in Phase IIB/III trials—and it is generally well tolerated, according to Dr. Proia.
Hsp90, a chaperone essential for the folding of JAK2, is the target of Synta Pharmaceuticals’ ganetespib.
Treating Alcoholism Like Cancer
The neurobiology of alcohol addiction is much more complicated than that of other drugs of abuse because it doesn’t have a well-defined site of action—alcohol is “not something that works on GPCRs or on dopamine transporters, for example, as cocaine does,” remarked Dorit Ron, Ph.D., professor of neurology at University of California San Francisco.
Although the mechanisms are different, the end result is the same—all drugs of abuse increase the levels of dopamine in the nucleus accumbens (NAc), a key component of the reward circuit—and “if you study addiction that’s usually the pathway where you start.”
Dr. Ron and her colleagues found that the PI3K/AKT/mTOR complex 1 (mTORC1) signaling pathway in the NAc is activated in response to alcohol, leading to translation of new synaptic proteins. “So we think this is a mechanism that can either lead to or maintain these phenotypes such as binge drinking, relapse, or seeking of alcohol.”
There are three drugs that the FDA has approved for treating phenotypes such as craving and relapse, “and they don’t work well,” Dr. Ron explained. “The problem with the drugs that we have now is that they basically shut off the reward pathway, so people don’t feel good; they basically don’t have the desire to do anything.”
But what if the same drugs that big pharma is developing for cancer could be used to treat alcohol abuse? As a proof of concept, Dr. Ron’s team showed that various inhibitors of the PI3K/AKT/mTORC1 pathway in the NAc decrease excessive alcohol intake and lessen other behaviors seen in preclinical rodent models of alcohol abuse.
On the flip side, researchers are also trying to understand whether alcohol use can increase the risk of cancer and act as a carcinogen. Details here.
In terms of anticancer agents for alcohol abuse, Dr. Ron pointed to Rapamycin, for example. “It does all the things that we want, but it really is very selective.” Although it’s an FDA-approved drug, because rapamycin is an immunosuppressant it won’t be used to treat alcohol abuse. But like many researchers at the conference, Dr. Ron is keeping her eye out for its derivatives, as well as other ways to interfere with intracellular signaling pathway, with fewer side effects.