October 1, 2012 (Vol. 32, No. 17)
William Downey
Dawn Miles
Over the past decade, planned average annual capacity growth in the biopharmaceutical contract manufacturing industry has slowed. This situation reflects both recent market conditions and a shift in investment priorities by biopharmaceutical contract manufacturing organizations.
CMOs have realigned their investment plans to focus on productivity improvements, lower costs, and better asset utilization.
This change in investment policy is documented in HighTech Business Decisions recent report, “Biopharmaceutical Contract Manufacturing: New Participants, Expanded Services and Emerging Markets.” Planned capacity increases, measured by bioreactor volume in liters, for both microbial fermentation and mammalian cell culture have slowed over the last seven years.
In 2005, CMOs expected to add capacity at a rate of 13% per year. Over the subsequent years, expected annual capacity increases have steadily declined. In 2011, CMOs planned to add capacity at a rate of 3% per year.
The CMOs interviewed for this article gave examples of the investments they are making to meet the current and future industry needs.
While new planned expansions have slowed, the CMO industry continues to take advantage of current biotech industry conditions. On July 31, CMC Biologics acquired Xoma’s large-scale manufacturing operations and affiliated assets in Berkeley, CA.
CMC Biologics has taken over the lease of buildings and three 2,750 L stainless steel bioreactors and two purification suites. CMC Biologics has already put a management team in place, and it expects the new facility to be fully operational by the end of this year.
Following the sale of its production facility, Xoma announced that it had entered into an agreement with Boehringer Ingelheim to manufacture gevokizumab, Xoma’s interleukin 1-beta allosteric modulating antibody. Boehringer Ingelheim will manufacture this product for both Xoma and its partner Les Laboratories Servier.
CMC Biologics’ acquisition deal with Xoma is the most recent involving biomanufacturing capacity made by a CMO in the past year and a half. Last year, Boehringer Ingelheim, and Fujifilm, and Gallus BioPharmaceuticals each made major biopharmaceutical manufacturing acquisitions from drug innovator companies.
Boehringer Ingelheim purchased Amgen’s Fremont, CA, biomanufacturing facility. Fujifilm bought Merck & Co.’s contract manufacturing business, and re-named it Fujifilm Diosynth Biotechnologies. Closely following these two acquisitions, Gallus BioPharmaceuticals purchased Centocor’s St. Louis, MO, manufacturing facility in May 2011.
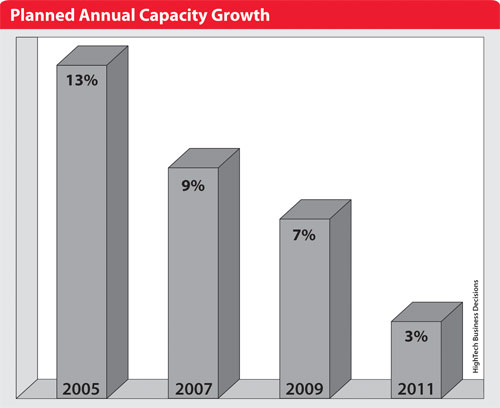
Planned annual capacity growth over last seven years
Capacity Investments
While CMOs continue to add capacity, they are generally doing so in smaller increments, and they are focusing on productivity and quality improvements to meet market demands. Most CMOs interviewed for this article are adding or have added single-use bioreactor capacity. Investing in single-use bioreactors allows for gradual increases in capacity at economically reasonable costs. Both large and small CMOs are installing single-use bioreactors.
For example, Fujifilm Diosynth Biotechnologies is expanding its mammalian cell culture offerings using single-use bioreactors.
“To meet market and customer demand we have significantly expanded our mammalian cell culture offering at both our U.S. and U.K. sites,” said Steve Bagshaw, managing director for the company. “Early this year at our Research Triangle Park site we brought on-line a single-use 1,000 L bioreactor that enables us to offer flexibility of operations to better serve the demands of companies requiring material for pre-clinical studies, early- to mid-phase clinical production, and beyond.”
Fujifilm Diosynth Biotechnologies is also building a cGMP mammalian cell culture facility at its Billingham, U.K., location, which will replicate its RTP mammalian cell culture operations.
In addition to implementing single-use bioreactor capacity, many CMOs are also implementing disposable technologies in downstream purification. The need for downstream improvements results in part from the work that led to higher cell culture titers over the past decade.
In many instances, higher titers caused the biomanufacturing production bottleneck to shift to downstream purification, thereby reducing the overall benefits available from implementing higher titer processes. For example, Boehringer Ingelheim and WuXi AppTec have implemented disposable technologies in both their upstream and downstream processes.
“Boehringer Ingelheim is adding fully disposable processes to our stainless steel capacity, which includes all upstream and downstream process steps,” said Julia Knebel, director marketing and communications, contract manufacturing business, biopharmaceuticals at Boehringer Ingelheim.
“A fully disposable process is one of the technology areas that has been fully evaluated and is no longer a future technology but more a present reality. Alternative technologies to chromatography are also very much in focus. Recent advances in membrane technologies such as membrane absorbers are having an impact throughout the purification process.”
“In the near term, membranes will improve the efficiency and effectiveness of process-based impurity removal such as virus and DNA removal, but in the future, may also impact specific product based impurity removal.”
WuXi AppTec recently completed construction of a new GMP manufacturing facility that employs disposable systems, including 1,000 L bioreactors.
“The entire operation employs disposable systems, both upstream and downstream, which greatly facilitates product changeovers, while reducing operational costs,” said David Fischer, business development manager, protein therapeutics at WuXi AppTec.
“We also will be expanding our disposable bioreactor capacity to 2,000 L by the end of the second quarter in 2013.”
In addition to investing in single-use technologies to meet customer demands for shorter project timelines, a few CMOs have been investing in fill-and-finish capacity. These CMOs are able to provide their clients with a one-stop-shop solution thereby reducing the logistics and coordination between two different CMOs. This helps speed delivery of clients’ drug product.
Laureate Biopharma, Boehringer Ingelheim and WuXi Apptec have recently made investments in fill-and-finish services.
Laureate completed improvements to its formulation and filling operations, including new analytics for formulation development and upgrades to its fill suites, resulting in improved process flow.
“Laureate has invested $8 million in its manufacturing facilities and equipment, quality systems, and analytical equipment,” said Lisa Cozza, vp of business development.
Other CMOs are also undertaking initiatives to improve product timelines for their customers. CMOs are adopting tools and techniques that will speed cell-line development, and facilitate their clients’ clinical studies. For example, CMC Biologics is offering faster cell-line development services.
“Our custom CHEF1® cell-line development platform provides expression solutions for both monoclonal antibodies and glycoprotein production,” said Claes Glassell, CEO. “The platform has been designed to deliver clonal research cell banks capable of producing more than 2 g/L of recombinant antibody in 12 weeks from transfection.”
CMC has also recently offered a new program to its clients that it reports delivers clinical-scale monoclonal antibodies in 12 months.
Also working to reduce project timelines for its clients, Gallus recently announced that it has begun to collaborate with Theorem Clinical Research to provide their clients with seamless integration between their CRO and the contract manufacturer. This collaboration streamlines the clinical studies activities with manufacturing development and production.
“This addresses a flaw in the current industry norm where CRO and CMO services are not linked and yet there is a vital role to play in delivering efficient and effective clinical development to clients,” said Mark R. Bamforth, president and CEO at Gallus Biopharmaceuticals.
New Capabilities
CMOs continue to implement new capabilities that either speed product development or improve product quality.
“Sandoz has successfully implemented its microbial autoprotease fusion expression technology (Npro) for two clinical candidates at 1,300 L and 3,000 L scale. The technology is available for partners of Sandoz,” said Friedrich Nachtmann, Ph.D., head of biotech cooperation at Sandoz.
Sandoz recently scaled-up the PEGylation of proteins to the 13,000 L scale and successfully integrated this process into its downstream manufacturing operations.

Sandoz recently scaled up the PEGylation of proteins to the 13,000 L scale.
In order to meet customers’ demands for better formulations, Wacker Biotech offers an alternative to PEGylation processes in cooperation with XL-protein.
“Many customers are currently looking for better formulations and, in particular, for technologies to improve the plasma half-life of biopharmaceuticals to reduce the dosing frequency,” explained Thomas Maier, Ph.D., managing director of Wacker Biotech.
“In contrast to the established chemical modification of the drug molecule with polyethyleneglycol (PEGylation), the new technology, PASylation®, offers a means to genetically fuse the therapeutic protein with a nonimmunogenic polypeptide comprising proline (P), alanine (A), and serine (S) residues and so increase the hydrodynamic volume.”
“PASylation has the advantage of avoiding an expensive and poorly selective chemical modification step during production and eliminates the need of additional purification. Moreover, unlike the chemical polymer PEG, the PAS amino acid sequences are biodegradable and therefore do not accumulate in cells or organs. We have already reached high production titers of 3 to 4 g/L for PASylated human growth hormone.”

Using its PASylation technology, an alternative to PEGylation, Wacker Biotech has reached production titers of 3 to 4 g/L for human growth hormone.
William Downey ([email protected]) is president and Dawn Miles is senior market analyst of HighTech Business Decisions.







