June 15, 2006 (Vol. 26, No. 12)
On-Line and Viable with Dielectric Spectroscopy
Cell culture and fermentation are used to produce pharmaceutical products or other molecules of interest. To achieve maximum efficiency or quality assessment during the synthesis of targeted products, on-line information about the active metabolic cell concentration is of crucial importance.
Although biomass is the catalyst of bio-production and its concentration is a key parameter in the reaction, bioreactors are commonly monitored with traditional pH, temperature, and dissolved oxygen sensors. In fact the optical technologies that have been proposed to monitor biomass suffer from a number of disadvantages such as sensitivity to cell debris and other media components or loss of linearity. Dielectric spectroscopy, or capacitance, has the advantage of only measuring the viable cell density.
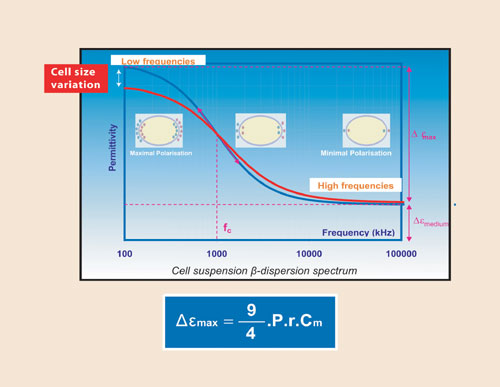
Fig.1: Cell suspension permitivity, a frequency-dependent signal described as the B-dispersion, r is the cell readius, Cm the membranes capacitance, and P the cell volume that corresponds with the cell biomass concentration
Theory of Dielectric Measurements
The limited ionic permeability of the plasma membrane gives living cells specific dielectric properties. At high frequencies, above 10 MHz, the ions’ movement amplitude is limited, and not significantly affected by the presence of the cytoplasmic membrane. A cell suspension has nearly the same overall electric properties as the suspending medium.
Permittivity at 10 MHz:
10 MHz = ε suspending medium
At lower frequencies, the displacement amplitude is higher, ions accumulate at the membrane, and the cells behave as tiny capacitors. The global charge accumulation is dependent on the viable cell density.
At low frequencies, when the cells are fully polarized, there is no further permittivity increase related to charge accumulation. The increase in permittivity from high to low frequencies has a sigmoidal shape and is known as the β-dispersion (Figure 1).
Viable cell density monitoring: measuring biovolume
The overall permittivity amplitude of the β-dispersion is proportional to biovolume, cell size, and membrane capacitance. In order to achieve high accuracy cell-density measurement, FOGALE Nanotech(www.fogale.com) is using a particular property at the critical frequency of the b-dispersionthe permittivity at fc is independent of cell size.
Permittivity at fc:
εfc = εbiomass + εsuspending medium
The permittivity difference measured between the critical frequency (fc) and 10 MHz results in high accuracy and very good linearity with off-line biovolume, even in permittivity changing media.
Δε(fc-10MHz) = εbiomass = k*biovolume
Biovolume is defined as the volume enclosed by intact cytoplasmic membranes (viable cell density); Biovolume=VCC*Vc; VCC: Viable cell count, and Vc: Average cell volume.
Using its dielectric-measurement technology FOGALE nanotech recently launched a new generation of on-line biomass-monitoring tools (Figure 2). The patented Biomass System is an affordable sensor for viable cell-density monitoring, and it is directly inserted into the bioreactor with in situ sterilizable standard 12-mm and 25-mm diameter probes to fit any bioreactor port. The probes’ design and components are ideal for working in a cGMP environment as the wetted material conform to FDA requirements (PEEK, 316-L stainless steel).
The Biomass System is useful for monitoring animal cells in suspension (including clumping cell lines) or attached to micro-carriers. It is also routinely used for yeast or bacteria batch and fed-batch processes with high biomass concentration up to 200g/L dry weight.

Fig. 2: FOGALE Biomass System
Accelerating Cell Culture Process Development
Example 1Sf9 Growth and Infection Process
Spodoptera frugiperda (Sf9) insect cells are used with the baculovirus expression vector system (BEVS) to express recombinant eukaryotic proteins or to produce baculovirus. When Sf9 cells are subjected to growth arrest after viral infection, this leads to an intrinsic death cascade process, therefore limiting the productivity of the culture and affecting downstream processing steps by increasing the amount of host cell proteins and debris in the system.
The on-line viable cell-density determination is used to better characterize the production process and its success. On-line permittivity is linearly related to viable cell density (biovolume) and allows clear real-time identification of the different stages of the culture (Figures 3,4)
The first is the exponential growth phase. The permittivity and viable cell count are well correlated because the cell diameter during growth phase is relatively stable.
The second is the infection phase. After baculovirus infection (50h), the cell division is stopped, and the permittivity increase (biovolume) depends essentially on the cell size, which strongly increases during this stage.
Next, is the production phase. After a plateau (first viral release) in the permittivity signal at 20h post-infection (p.i.), the viable cell count, biovolume, and cell diameter remain relatively constant.
Finally, we reach the death phase. The permittivity drops at 50h p.i. correlating with the decrease in average cell diameter and viability.
Example 2CHO Batch Culture
Figure 5 demonstrates how the on-line permittivity signal can be used to monitor viable cell density during a 2-L CHO batch culture. The Biomass System (fitted with a 12-mm diameter sensor) permittivity is providing a linear correlation with viable cell count over the complete time course of the culture.
In addition, changes in cell metabolism can be monitored by means of the permittivity measurement (Figure 6). The first drop in permittivity corresponds to a metabolic shift where glutamine becomes a limiting nutrient. The oxygen uptake rate also decrease significantly. Alanine is accumulating until glutamine is depleted and subsequently consumed. The second drop is correlated to the exhaustion of alanine and glucose that marks the beginning of the death phase. The same patterns are obtained from a larger scale culture with a 25-mm diameter sensor.
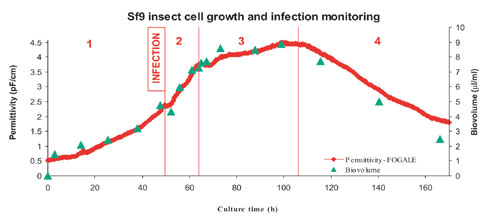
Fig.3: Sf9 cell growth and infection process monitored by online permittivity
Conclusion
Permittivity-based measurement is an accurate method for on-line viable cell-density determination. It offers a unique opportunity for better cell culture process understandings and faster developments. This technology provides a reliable tool for PAT application in a production environment.
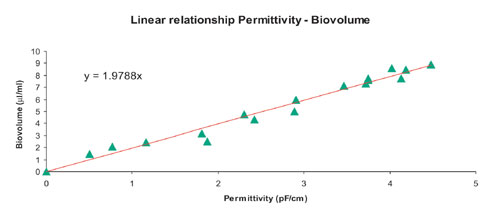
Fig.4: Biovolume versus permittivity during Sf9 growth and infection process
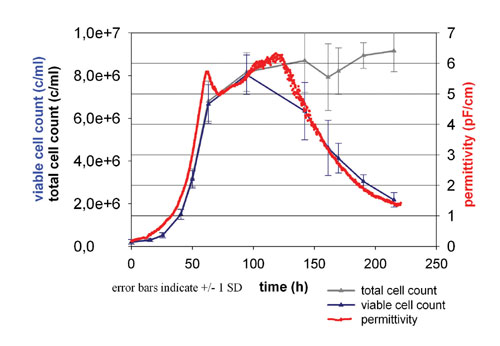
Fig.5: Permittivity, viable and total cell counts during a CHO 20-L Bath culture
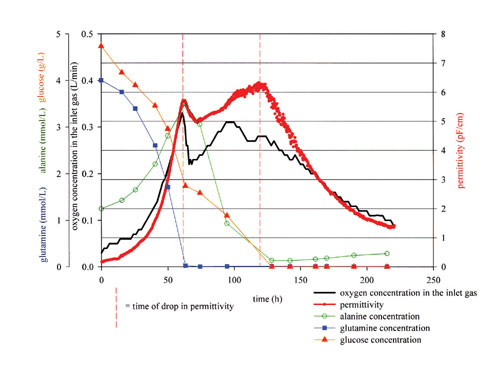
Fig.6: Permittivity, volumetric O92) uptake rate, and main nutrient concentrations during a CHO 2-L batch culture







