April 15, 2012 (Vol. 32, No. 8)
Michael D. O’Neil
The group of seven transmembrane-spanning G protein-coupled receptors (GPCRs) represents the largest superfamily of receptors in the human genome. GPCRs are involved in virtually all physiological processes and represent the targets for at least 30% of all current medicines. GPCRs sense molecules outside the cell and, in response, activate intracellular signal transduction pathways and, ultimately, cellular responses.
Keystone Symposium’s “G Protein-Coupled Receptors: Molecular Mechanisms and Novel Functional Insights” meeting held recently addressed the latest developments in this exciting area.
Stephen Rees, Ph.D., vp of screening sciences and management at AstraZeneca, described the results of a recent effort to identify native ligands for orphan GPCRs. Such efforts are quite important, Dr. Rees asserted, because “GPCRs are the most important target class for the discovery of molecules for pharmacological intervention in disease, both in terms of the proportion of marketed drugs with activity against this target class and in terms of industry revenue.” It therefore seems likely that many of these orphan GPCRs will also prove to be of medical significance.
Despite extensive efforts, Dr. Rees said, there remain over 100 GPCRs for which the native ligand has not been conclusively identified. Ligand identification would help enable target validation and would also enable drug screening assays for the GPCRs.
While Dr. Rees was at GlaxoSmithKline he was involved in a collaboration with DiscoveRx and Medical Research Council Technology (MRCT) to screen 90 orphan GPCRs against a comprehensive panel of 10,000 known bioactive molecules for their ability to interact with the GPCRs to selectively regulate the beta-arrestin signaling cascade. This method was chosen because it is relatively simple to measure, and it was believed that many, if not all, GPCRs affect this signaling cascade.
The result of the study was disappointing, Dr. Rees reported, as native ligands were identified for only 3 of the 90 orphan GPCRs. Dr. Rees said that this may indicate that there may be only a small number of GPCRs that selectively regulate the beta-arrestin signaling cascade, and that ligands for the remaining orphan receptors remain to be discovered. Nevertheless, native ligands for three previously orphan GPCRs were identified, and two of these GPCRs are now the subjects of ongoing drug discovery efforts at MRCT.
Oncogenic and Pro-Metastatic Signaling Circuitries
J. Silvio Gutkind, Ph.D., chief, oral and pharyngeal branch, National Institute of Dental and Craniofacial Research, NIH, emphasized the growing awareness that GPCRs can play key roles in cancer and that these molecules represent a new source of anticancer targets that may be exploited. In particular, he noted that in the last three years cancer genome sequencing results have revealed an unexpectedly high rate of GPCR gene mutations (5% to 30%) in some of the most prevalent human cancers.
In fact, a Genentech study published in 2009 indicated that approximately 20% of cancer mutations actually occur in G protein or GPCR genes. Activating mutations in Gαs genes have been observed in multiple adenomas and genes for both Gαq family members (GNAQ and GNA11) and also were recently identified in approximately 80% of uveal melanomas, where these genes are considered the driving melanoma oncogenes.
A direct link between GPCRs and viral-associated malignancies was previously established (1998–2003) by Dr. Gutkind and his colleagues with the identification of a constitutively active oncogenic GPCR (vGPCR) coded by the Kaposi’s sarcoma (KS)-associated herpes virus (KSHV) that is the infectious cause of KS.
vGPCR activates an intricate network of molecular-signaling events, driving both the aberrant growth of endothelial cells and the paracrine transformation of endothelial-derived cells expressing KSHV latent genes. vGPCR can, by itself, induce KS lesions, Dr. Gutkind noted.
Dr. Gutkind said that angioproliferative tumors induced by KSHV have been successfully treated with rapamycin, providing direct evidence of the clinical activity of mTOR inhibition in human cancers. However, prolonged mTOR inhibition in immunocompromised patients, such as those with AIDS-KS, may raise concerns.
Therefore, Dr. Gutkind’s group recently investigated whether KSHV oncogenes (specifically vGPCR) deploy cell type-specific signaling pathways activating mTOR and if such specific pathways could be targeted to halt KS development while minimizing immune-suppressive effects. The group determined that the PI3Kγ isoform is strictly required for signaling from the vGPCR oncogene to the Akt/mTOR signaling pathway. The group then generated genetic and pharmacological evidence that PI3Kγ may represent a suitable therapeutic target in KS.
Martin Lohse, M.D., professor of pharmacology at the University of Würzburg in Germany and chairman of the Rudolf Virchow Center, discussed his group’s efforts to carry out studies of single GPCR molecules. Dr. Lohse said that for a decade his group has studied cell receptors using fluorophores in order to directly observe the receptors, how and where they move, how fast they turn on and off, and how they trigger cell reactions. This work is now going in two directions—first in the direction of drug development and drug screening using optical tests, and secondly, in the direction of single-molecule studies.
Dr. Lohse believes the single-molecule studies have the potential to yield great insight into the workings of GPCRs. He drew the analogy with the progress allowed by the development of the patch-clamp technique in electrophysiology, which enabled the study of single-ion channels in cells and proved their involvement in fundamental cell processes.
Previously, scientists have only been able to look at the behavior of GPCRs in aggregate, and this behavior may be the sum of many different individual reactions.
Increasingly, Dr. Lohse said, the data on the overall behavior of GPCRs and signaling proteins are being complemented by studies of individual GPCRs and signaling proteins, which show distinct and specific behavior of individual proteins in terms of their mobility and protein-protein interactions.
These studies are made possible by using highly fluorescent labels combined with single particle tracking. Together, these experiments show that GPCRs have very distinct properties, ranging from receptors that act in strict linear chains to those that exist and function in large protein aggregates that allow complex interactions and signaling behavior.
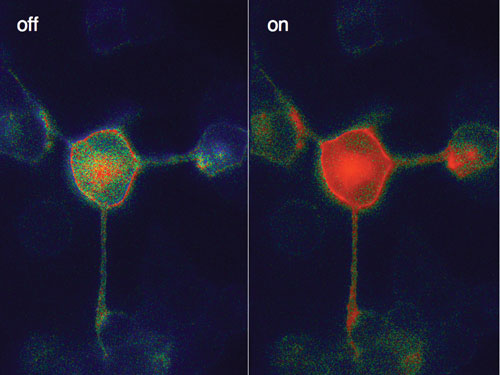
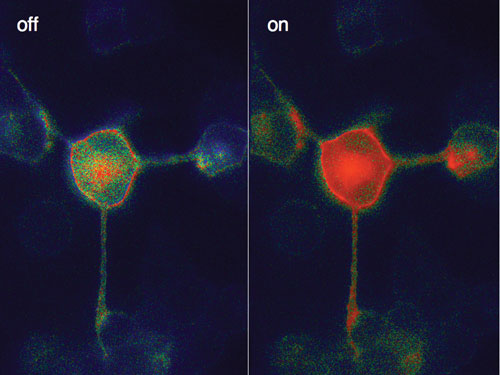
Researchers at the University of Wurzburg have studied cell receptors using fluorophores in order to directly observe the receptors, how and where they move, how fast they turn on and off, and how they trigger cell reactions.
Ruben Abagyan, Ph.D., professor at the Skaggs School of Pharmacy & Pharmacology at the University of California, San Diego, described efforts to expand the x-ray crystallographic structures of the dozen GPCRs determined so far to predictive models of hundreds of GPCRs in different conformational states.
Dr. Abagyan noted that with recent work the community now has the crystal structures for nine GPCRs and will soon have the crystal structures for two or three more. This represents an “incredible advance,” he said, over a decade ago when we had the crystal structure of rhodopsin, but of no other GPCR. Nevertheless, there are estimated to be approximately 1,000 different GPCRs in the human genome, and so the current structural knowledge of a handful of GPCRs represents only 1% of the total.
Dr. Abagyan emphasized that improved modeling techniques promise to expand the scope of GPCR proteins whose structures are understood, as well as increase the understanding of how a given GPCR binds to chemically different agonists, antagonists, inverse or partial agonists, and allosteric modulators.
In particular, he described three different levels of structure-ligand binding computational predictions that can be carried out today. The easiest approach is if the crystal structure of the GPCR is known. In such cases, Dr. Abagyan showed that a virtual ligand screening approach using the latest docking tools and ICM (Internal Coordinate Mechanics) software demonstrated an over 90% success rate in predicting the correct ligand binding pose in a docking exercise to a single cognate pocket determined by crystallography, and 90% reliability in a more realistic cross-docking exercise if multiple experimental pocket conformations were considered.
This represents “an outstanding result,” Dr. Abagyan said, when compared with the typical cross-docking success rates of 30% to 70% obtained with previous approaches. Moreover, in addition to the docking pose of active molecules, he showed for two better-characterized GPCRs that the docking scores are now capable of separating thousands of actives from inactive molecules.
The second, more challenging approach applies when the GPCR crystal structure is not known, but that of a homologue is. In such cases, knowledge of previously discovered receptor ligands provides key information that can be used for helping the modeling process and improving docking and screening performance of the receptor.
Dr. Abagyan demonstrated how the so-called “ligand-guided optimization of homology models” improves the initially inaccurate models of the ligand-binding pockets into an ensemble of highly predictive models. The new implementation, abbreviated as Alibero or LiBERO, improves the outcome of the procedure, Dr. Abagyan said.
The third and most difficult approach is that for the overall model of a GPCR or an allosteric pocket that forms upon binding. For these cases, Dr. Abagyan’s group and his colleagues at Molsoft have developed an improved homology modeling approach that includes several essential elements: (i) a new force field that was tested for a better ability to predict loop conformations, (ii) a new membrane implicit solvation model, and, (iii) an adequate atom-level transfer of the distance restraints from a template to the model.
The approach can be further assisted with what Dr. Abagyan termed as “fumigation” because it offers the potential of opening up the binding site for interaction with a potential small molecule modulator.
Fluorescent Ligands
Stephen Hill, Ph.D., head of the School of Biomedical Sciences and professor of molecular pharmacology at the University of Nottingham Medical School, described the effective use of fluorescent ligands to investigate the function of GPCRs at the single living cell and single-molecule levels.
Dr. Hill said that this important advance has been made possible by progress in confocal microscopy, quantitation of fluorescence intensity at the cell membrane, and the development of fluorescent ligands that retain their pharmacological activity and whose fluorescence is much brighter when the ligand binds to a cell surface receptor than when free in solution.
Dr. Hill noted that the ability to work at the single-molecule and single-living-cell levels “will be critical when working with limited numbers of cells, such as is often the case in clinical investigations.”
In addition, the approach allows detailed kinetic studies to be undertaken with the receptor in its native environment within the cell membrane. Also, the increased resolution and temporal capability of these techniques can be applied to native cells endogenously expressing the receptor of interest.
Using live CHO cells bearing recombinant human adenosine A1 or adenosine A3 GPCRs, Dr. Hill demonstrated that a receptor-specific agonist or antagonist could inhibit the binding of a fluorescent ligand to cell surface receptors at the single-cell level. He further demonstrated that this approach could be used in a 96-well plate for high-throughput screening with a confocal imaging plate reader to investigate how well a nonfluorescent drug might inhibit GPCR-ligand-specific fluorescence in live cells.
The approach could also be used to quantitate the effect of small molecular fragments on fluorescent ligand binding in fragment-based drug discovery, he noted. This successful use of fluorescence with live cells represents a major advance over the current approach of radiologic labeling of GPCRs in membrane homogenates, he added.
Dr. Hill went on to show that the same fluorescent approach could be used to label adenosine A3 receptors in live human neutrophils where these GPCRs are endogenously expressed. In neutrophils, the adenosine A3 receptors are known to coordinate chemotaxis and, not surprisingly, Dr. Hill said, observation of the fluorescent signals indicated that the adenosine A3 receptors were located at the leading edge of the neutrophil.
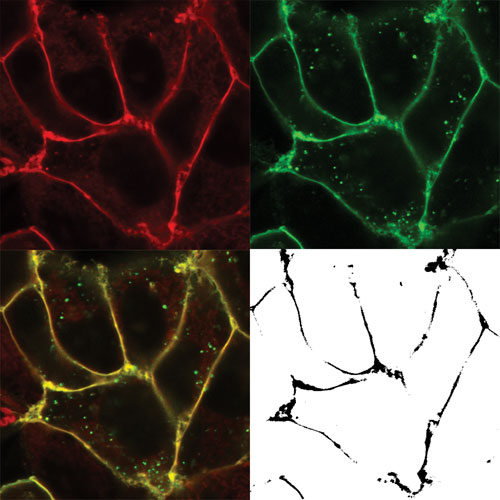
Co-localization of ligand and GPCR: Human embryonic kidney cells (HEK-293T) that express a SNAP-tagged human adenosine-A3 receptor (green, upper right quadrant). The cells have been incubated with red fluorescent adenosine-A3 receptor antagonists (red, upper left quadrant). The bottom left quadrant is the merged image (yellow indicates co-localization of ligand and receptor). The bottom right quadrant image is the co-localization analysis, indicating black pixels where ligand and receptor are co-localized. [Stephen Hill, University of Nottingham Medical School]