September 1, 2012 (Vol. 32, No. 15)
New Biomarker Testing Capabilities Helping to Change the Face of Patient Management
Physicians depend on tests for cancer biomarkers at various disease stages to help them diagnose, monitor, predict treatment or prognosis, and also predict risk for their patient’s particular cancer. Laboratory testing for cancer biomarkers spans multiple clinical detection platforms and includes traditional mainstays like immunoassay, immunohistochemistry, and flow cytometry and also the newer molecular-based platforms like PCR, microarrays, and sequencing.
Frost & Sullivan sized the U.S. cancer biomarker testing market at $7.86 billion in 2011 and expects this to reach $11.46 billion by 2017 with positive growth driven by incorporation of companion diagnostics (KRAS, EGFR, BRAF V600E) into clinical guidelines and then rapid development of clinical next-generation sequencing (NGS).
The molecular-based cancer biomarker testing segment, including single-mutation companion diagnostics, multiplexed array, and other genomic analysis, were only 4% of total market revenues in 2011, but this will likely reach 9.4% by 2017 from 22.9% annual growth.
A recent Frost & Sullivan analysis of the U.S. cancer biomarker testing market produced a number of predictions about what cancer testing, new test development, and patient management will look like in the future.
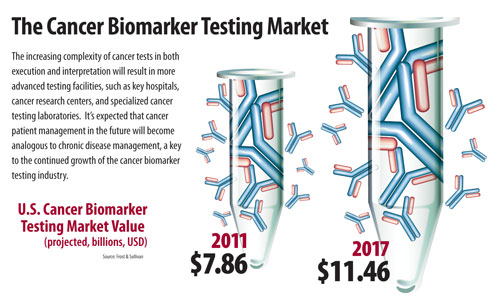
The Cancer Biomarker Testing Market
Cancer Biomarker Tests Will Exhibit Greater Multiplexing and Incorporate Multiple Biomarker Types
Some single-gene mutation tests will be replaced by cost-efficient multiplexed tests. We are already seeing potential cases for this, including Bio-Reference’s Onkomatch test that matches effective therapeutic agents (either currently marketed or in a clinical trial) to the tumor molecular profile of lung cancer patients. The Onkomatch test evaluates 68 key mutations including EGFR, BRAF, and KRAS at a list price of $995; a far better price point than running EGFR and KRAS individually, which can total more than $1,000 at other laboratories.
Specific NGS applications, namely exon capture followed by targeted NGS, are likely to drive test costs to less than $1,000. NGS testing service will become more feasible for clinical laboratories to integrate within the next one to two years. The caveat to having more multiplexed tests will be effectively changing test ordering behavior by clinical decision-makers through adequate education, especially considering that the testing of certain gene mutations and even testing methods are recommended in clinical guidelines, but not regarding multiplexing tests.
Other resistance would be logistical, such as if the multiplexed test is not offered at their preferred or contracted laboratory. While it would be logical to predict that future cancer biomarker tests will also combine multiple biomarker types in signatures that offer increased test sensitivity and specificity, epigenetic and miRNA targets show promise as highly specific cancer biomarkers.
More Cancer Biomarker Tests Will Originate from Multicenter Research Consortiums and Cancer Testing Will Become Centralized
Large multicenter research efforts such as those involving designated NCI centers provide advantages in specimen and resource pooling. Furthermore, the high quality of the clinical research and collaboration will likely translate into clinically valuable and well-validated tests. Evidence of this trend can be seen from the Lung Cancer Mutation Consortium, the largest national initiative to prospectively study non-small-cell lung cancer tumors and match them to the best therapies.
The resulting genetic-based assay technology was licensed and commercialized by Bio-Reference Laboratories and the protein-based assay technology was licensed by SomaLogic for commercialization. This example also illustrates the increasing complexity of cancer tests in both execution and interpretation. As a result, advanced cancer testing will likely be centralized to key hospitals, cancer research centers, and specialized cancer testing laboratories.
Cancer Will Resemble Chronic Disease Management
Tumor tissues are always changing and the existence of heterogeneous cell populations enables rapid adaptation to changes in its microenvironment (such as drug treatment). This is the current model for tumors that stop responding to therapy. Tools for cancer patient management in the future will include targeted therapy options (and associated companion diagnostics) and tests that provide continuous feedback about tumor response, ideally noninvasively.
When therapies become ineffective, and the tumor develops resistance, new mutations and key pathways that are driving tumor growth must be reassessed. Unfortunately, dynamic capture of the tumor’s molecular profile would increase the number of biopsies; however, this would create a market opportunity for noninvasive tests to monitor the molecular changes driving the pathology.
Circulating tumor cells seem to be possible candidates for noninvasive tumor molecular profiling but leave much to be desired since their presence means the tumor has metastasized and is in a late stage. It is clear that cancer patient management will not be fully addressed by a single test or a single platform and additional monitoring tests will be needed in order to guide necessary changes in treatment that effectively shrink the tumor or control its growth.
If all of these elements fall into place, cancer patient management in the future will become analogous to chronic disease management.
Winny Tan, Ph.D., is an industry analyst in the life sciences group at Frost & Sullivan. Contact Britni Myers ([email protected]) for more information.



