September 15, 2008 (Vol. 28, No. 16)
New Technology Was Designed for Screening and Profiling Applications
DiscoveRx has developed a cell-based nuclear hormone receptor (NHR) assay using its enzyme fragment complementation (EFC) technology that provides notable benefits for screening and profiling applications. The assay is based on detection of protein-protein interactions between an activated, full length NHR protein and a nuclear fusion protein containing steroid receptor co-activator peptide (SRCP) domains with one or more canonical LXXLL interaction motifs.
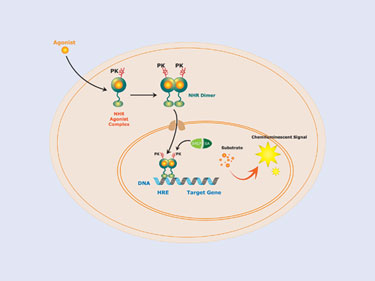
The NHR is tagged with the ProLink™ component of the EFC assay system, and the SRCP domain is fused to the enzyme acceptor component (EA) expressed in the nucleus. When activated, the NHR will associate with the SRCP domain, and complementation will occur, generating an active b-Gal enzyme and production of chemiluminescent signal (Figure 1).
Benefits associated with this approach include reduced compound incubation times, direct measurement of the NHR target, use of a full-length human NHR sequence, and the ability to select novel compound classes based on disruption of protein-protein interactions.
Nuclear hormone receptors represent a highly druggable class of target proteins that are most commonly analyzed using a reporter gene assay format. This presents challenges due to high background activity of certain NHRs, as well as nonspecificity in the response due to long incubation times. Furthermore, developing the assay requires the generation of a reporter gene construct and knowledge of the required DNA-binding sites for the receptor. Some of these issues can be addressed with direct protein analysis in the cell, most often with imaging, but that typically requires implementation of an HCS system, adding complexity to the assay and reducing throughput.
DiscoveRx’ PathHunter NHRPRO Assay, a simple, homogenous cell-based assay, was developed for screening and profiling applications. It is specific, sensitive, and amenable to high-throughput strategies while retaining the use of a full-length receptor.
CHO-K1 cells expressing a full-length thyroid hormone receptor alpha (THRa1, NR1A1) fused to a ProLink fragment of b-Gal were retrovirally infected with a SRCP-EA construct. Forty-eight hours after infection, the cells were gently removed from the plate using trypsin, replated, and grown in selective media for one week. The cells were trypsinized and plated in a 20 µL well of serum-free media at a density of 10,000 cells per well in a 384-well plate 24 hours prior to the experimental procedure.
CHO-K1 cells expressing SRCP-EA were infected with retrovirus expressing the NHR-PK of interest (either PPARg or ERRa). Forty-eight hours after infection, the cells were placed under G418 selection to select those cells expressing the NHR-PK construct. Seven days later, the cell population was placed into clonal dilution to generate single cell clones. The clones were tested functionally for response to the appropriate agonist for the NHR-PK expressed.
The NHRPRO cells were subjected to either agonist or inverse agonist (5 µL/well) treatment for six hours at 37°C. Following incubation, 13 µL of the PathHunter detection reagents were added to each well and incubation continued for one hour at room temperature (20–22°C). After one hour, the cells were analyzed using a 700 nM CL filter on a Victor2 1420 multilabel counter at a read setting of one second per well.
Figure 1
Results and Discussion
The clone of THRa-PK and SRCP-EA shows the expected nuclear staining of the exogenously expressed thyroid receptor (Figure 2a). We test the same cell pool in the PathHunter NHRPRO assay with a known THRa agonist, T3 (Triiodo-L-Thyronine). A significant sixfold response in signal after six hours of treatment was observed, indicating that THRa behaves similarly to the endogenous THRa by interacting with SRCP after treatment with T3 (Figure 2b). The PathHunter NHRPRO assay was shown to be more time efficient than current NHR assays and responds characteristically to agonist.
In the second experiment, we treated a cell line coexpressing ERRa and SRCP-EA with XCT790, a known inverse agonist, overnight at 37°C and assayed the results. ERRa responds characteristically by dissociating from SRCP-EA. This decrease in interaction results in a 2.5-fold drop in signal (Figure 3). PathHunter ERRa assay is able to detect a robust decrease in NHR and coactivator association.
Finally, we demonstrated that the PathHunter PPARg NHRPRO assay is specific and can be used to reveal novel compound classes based on protein-protein interactions. We tested a panel of 76 nuclear receptor ligands preformatted in a 96-well plate. As expected, we found that three previously known PPARg agonists showed strong activity in our assay. The PPARg NHRPRO cells in wells containing Pioglitazone HCl, GW7647, or trogliotazone compounds demonstrated a 16.7, 16.0, or 10.7-fold increase in activity, respectively.
The PathHunter NHR assay exposes compounds that activate PPARg. We found that some compounds that act as antagonists for other NHRs serve to weakly activate PPARg in our assay. For example, mifeprestone, a progesterone receptor antagonist, and tamoxifen, an estrogen receptor antagonist, both weakly activate PPARg (Figure 4). There has been precedence for antagonists acting as agonist for different NHRs and vice versa, so this was not unexpected. The PathHunter NHRPRO assay uncovers classes of compounds for NHR activity based on protein-protein interaction.
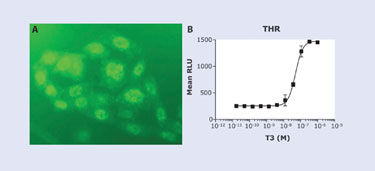
Figure 2
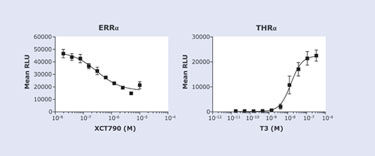
Figure 3
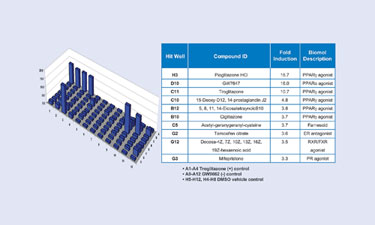
Figure 4
Mahesh Mathrubutham, Ph.D., is principal scientist, Patricia Whaley, Ph.D., ([email protected]) is technical marketing manager, Tom Wehrman, Ph.D., is director of cell biology, and Keith Olson, Ph.D., is vp of R&D at DiscoveRx. Web: www.discoverx.com.