February 15, 2007 (Vol. 27, No. 4)
Ramin Saberi
MicroRNA Detection Combining xMAP® and Locked Nucleic Acid (LNA™) Technology
MicroRNAs are a class of noncoding regulatory RNA molecules that affect gene expression by binding to 3´-untranslated regions of messenger RNAs (mRNAs). Although recently identified as a new class of molecules, initial studies indicate that microRNAs may regulate a significant number of genes within the genome—as many as one-third—and comprise a hidden level of gene regulation that influences a wide range of biological activities and cellular processes, such as cellular proliferation, maintenance, and death; differentiation of cell lines; developmental patterning and timing; and carcinogenesis. Recent findings further suggest that various diseases and conditions are associated with specific microRNA patterns, which may lead to the development of targeted treatments.
As the number of studies involving microRNAs increases, researchers are progressively more interested in accurate, easy-to-use technology that is specifically designed for microRNA analysis; however, microRNA analysis presents several challenges because the microRNAs are short sequences ranging from 17–23 nucleotides in length that are highly similar to each other in sequence with inherently different melting temperatures. The unique combination of a bead-based array and Tm-normalized locked nucleic acids offers a solution for these challenges.
Luminex (www.luminexcorp.com) recently introduced FlexmiR™, a line of products designed specifically for microRNA analysis. FlexmiR was developed by Exiqon (www.exiqon.com) in collaboration with the Luminex Bioscience Group. The FlexmiR microRNA products combine Luminex’ xMAP multiplexing technology with Exiqon’s Locked Nucleic Acids (LNA™) to deliver highly specific results in a simplified workflow.
The favorable reaction kinetics of the liquid bead array delivered by xMAP technology gives faster, more reproducible results than with solid, planar arrays. This “liquid array” approach also offers excellent manufacturing and assay standardization due to the nature of the microspheres when compared to competing flat arrays, which are limited by solid-phase kinetics.
LNA is a conformationally restricted nucleic acid analogue in which the ribose ring is “locked” with a methylene bridge connecting the 2´-O atom with the 4´-C atom, which increases the melting temperature of the nucleic acid duplex by 2–8°C per LNA monomer when integrated into one strand. The FlexmiR microRNA products incorporate LNA technology within the oligonucleotide capture probes to greatly increase the affinity of the probes for their complementary mature microRNA targets leading to significant increases and uniformity of assay specificity.
The FlexmiR microRNA detection panels for human and mouse/rat targets give researchers the ability to measure the expression of microRNA sequences from the public miRBase database using total RNA samples without the need for RNA size fractionation or amplification. The general flow of FlexmiR products allows researchers to biotinylate the 3´ end of total RNA, followed by a hybridization step where the labeled microRNA hybridizes specifically to LNA capture probes coupled to xMAP microspheres. The detection of the biotinylated microRNA is achieved by the reaction with Streptavidin-Phycoerythrin (SA-PE) and final read of the samples in a standard 96-well plate on a Luminex analyzer.
The experiments described here examine the capability of FlexmiR microRNA products to discriminate between two closely related microRNA family members. The capture probes have been Tm-normalized to hybridize optimally under the designed conditions by varying the LNA content and the length of the capture probes.
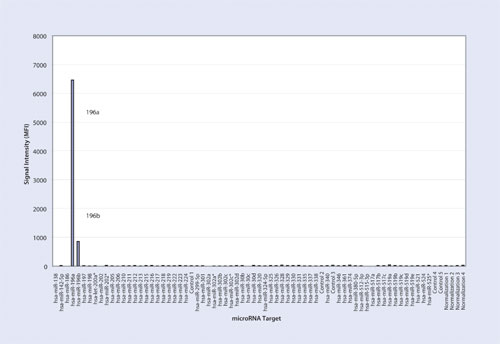
Figure 1
Experimental Design
Three experiments were designed to examine the discrimination capability of FlexmiR microRNA capture probes using two closely related microRNA family members, hsa-miR-196a and hsa-miR-196b from miRBase version 8.0. These two microRNAs are 21 bases long and differ by only a single nucleotide at position ten from the 3´-end:
hsa-miR-196a (5´-UAGGUAGUUUCAUGUUGUUGG -3´)
hsa-miR-196b (5´-UAGGUAGUUUCCUGUUGUUGG-3´)
The microspheres used in the experiment were from one of the available microsphere pools in the FlexmiR MicroRNA Human Panel, which contains 73 different microsphere sets coupled to different LNA-spiked capture probes specific for 73 different targets, including one set specific for hsa-miR-196a and another specific for hsa-miR-196b. The experiments were executed against synthesized DNA oligonucleotides with corresponding sequences to hsa-miR-196a and hsa-miR-196b with 5´ biotin modification.
The first experiment evaluated the microsphere pool against the hsa-miR-196a oligonucleotide individually at 5 femtomole (fmol). The second experiment evaluated the microsphere pool against the hsa-miR-196b oligonucleotide individually at 5 fmol. The third experiment involved mixing both the hsa-miR-196a oligonucleotide and hsa-miR-196b oligonucleotide each at 5 fmol and evaluated this combination against the pool of 73 microsphere-LNA probe sets.
For each experiment, the FlexmiR MicroRNA Human Panel Instruction Manual protocol was followed to perform hybridization of the synthetic oligonucleotide(s) to the LNA-doped capture probes coupled to the xMAP microspheres. Detection of the biotinylated oligonucleotides was achieved by the reaction with the Reporter Molecule, streptavidin-phycoerythrin conjugate, and final read of the samples in a microtiter plate on a Luminex 200 analyzer with Luminex IS Software Version 2.3. Samples were run in triplicate and an averaged value was reported.
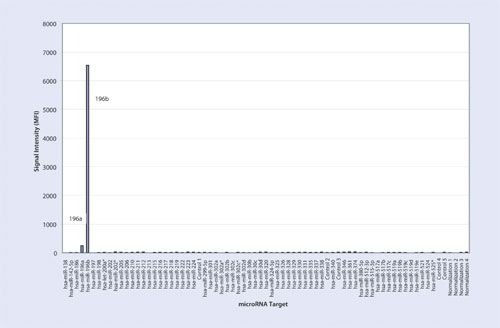
Figure 2
Results
The first experiment resulted in 6,479 Median Fluorescence Intensity (MFI) for the hsa-miR-196a oligonucleotide at 5 fmol with a signal of 874 MFI for hsa-miR-196b (Table and Figure 1). The second experiment yielded 265 MFI for hsa-miR-196a and 6,556 MFI for hsa-miR-196b at 5fmol (Table and Figure 2). The third experiment resulted in 6,776 MFI for hsa-miR-196a oligonucleotide and 7,684 MFI for hsa-miR-196b oligonucleotide each at 5 fmol (Table and Figure 3). For all three experiments, the measured signals remained below 70 MFI for all 71 remaining LNA capture probes coupled to microspheres.
Signal intensity of the perfect match capture probe was more than seven times higher than the single base mismatch capture probe for Experiment 1 (Figure 1) and it was about 25 times higher for Experiment 2 (Figure 2). In the presence of both targets, signal intensity of hsa-miR-196a oligonucleotide and hsa-miR-196b oligonucleotide had a coefficient variance of 3% and 11% respectively when compared to the signals of each target when they were run individually in Experiments 1 and 2.
All three experiments were repeated and the results were reproducible.
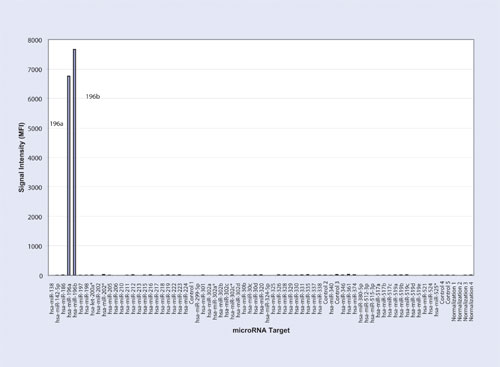
Figue 3
Conclusion
The potential for the FlexmiR microRNA product line lies in its unique combination of LNA and multiplexing technology, which provides the ability to offer quick and highly specific detection of microRNA targets in a simple workflow. These experiments, summarized in Figure 4, demonstrate that adjusting LNA content and the length of the capture probes enables Tm normalization of capture probes and provides normalized hybridization conditions suitable for all microRNAs.
Using LNA-modified capture probes with a uniform melting temperature (Tm) results in FlexmiR’s sensitive and precise detection of two closely related targets—even targets that are different by only a single nucleotide—which provides solutions to the challenges of discriminating short sequences with varying melting temperatures and high degrees of similarity.
The database of identified microRNA is growing rapidly. As the scientific community studies various microRNA sequences and begins to focus on critical, relevant microRNA, it will need technology that can help focus studies on the diseases and conditions of interest and that can easily expand or subtract feature sets. Hard-coded planar arrays will be at a disadvantage in offering the customization needs of emerging microRNA research.
The ability to customize a set of microRNAs while maintaining high specificity and simple workflow attributes should prove useful to those interested in focusing research to specific microRNA targets or patterns.
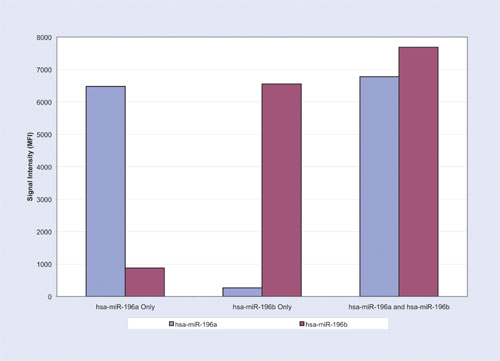
Figure 4