October 15, 2007 (Vol. 27, No. 18)
Vera Rakhmanova Ph.D.
Rich Meyer Ph.D.
Xiaohe Tong
Use of a Long-wavelength Fluorophore to Detect the Activity of MMPs
The latest advances in genome sequencing indicate proteases occur naturally in all organisms and account for about 2% of the human genome and 1–5% of genomes of infectious organisms. The human genome sequence has revealed more than 500 genes that encode proteases or protease-like proteins, with a large number being associated with tumor processes. Among these are the matrix metalloproteinases (MMPs), a group of enzymes that degrade the extracellular matrix and process bioactive mediators.
MMPs are zinc-dependent endopeptidases that share common structural and functional elements. Based on their role in normal and pathological processes, including embryogenesis, wound healing, inflammation, arthritis, and cancer, MMPs have been chosen as therapeutic targets for the treatment of many diseases.
FRET Pairs Used for Protease Assays
Various assays, such as zymography, ELISA, and peptide-based activity assays, have been developed to facilitate research and drug discovery for MMPs. Zymography is a relatively low-throughput method, while ELISA does not provide MMP activity information. In the last decade or so, the use of fluorescence or Forster resonance energy transfer (FRET) technology has made the continuous assay of protease activity and high-throughput screening of protease inhibitors faster and easier.
FRET relies on the distance-dependent transfer of energy from a donor molecule to an acceptor molecule. In general the donor and acceptor moieties are different, in which case FRET can be detected by the appearance of fluorescence of the acceptor or by quenching of donor fluorescence. The donor probe is always a fluorescent molecule. Because not all acceptor moieties are fluorescent, they can be used as fluorescence quenchers. Protease assays usually involve a fluorescent donor moiety on one terminus and a quenching acceptor molecule on the other terminus, separated by a peptide containing the protease cleavage sequence.
Appropriate donor/acceptor pairs should have enough spectral overlap for efficient energy transfer to take place yet have enough of a difference in spectra as to be distinguishable from one another. 5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid (EDANS, Ex/Em=340 nm/490 nm) is often used as a donor in conjunction with the quencher 4-[4-(dimethylamino)phenylazo]benzoic acid (DABCYL) in the development of FRET probes, as is (7-methoxycoumarin-4-yl)acetyl (Mca, Ex/Em=328 nm/393 nm) and the N-2,4-dinotrophenyl quencher.
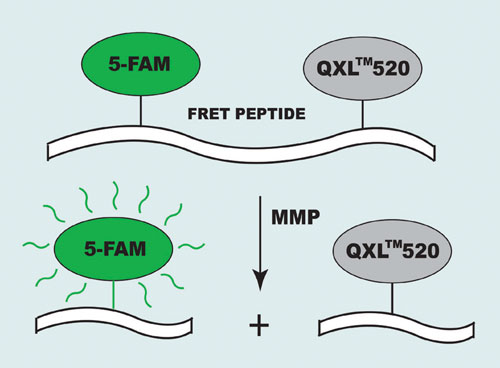
Their short absorption wavelengths and low extinction coefficients, however, create some limitations for MMP inhibitor screening. To eliminate these obstacles AnaSpec (www.anaspec.com) designed a series of long-wavelength 5-FAM/QXL™520 FRET substrates for the assay of MMPs. In the intact FRET peptide, the fluorescence of 5-FAM (5-carboxyfluorescein) is quenched by QXL 520. The cleavage of FRET peptide by the enzyme results in release of the donor from the acceptor. The fluorescence of 5-FAM is restored, which can be monitored at Ex/Em=490 nm/520 nm (Figure 1).
The QXL 520 quencher, a nonfluorescent dye, offers several advantages. The absorption spectrum of QXL 520 overlaps with almost the entire emission spectrum of 5-FAM thereby providing efficient quenching. The hydrophilicity of QXL 520 results in better solubility of the peptide substrate. Among the fluorescent donors, 5-FAM has much better brightness than EDANS and Mca. The extinction coefficient of 5-FAM is 13-fold higher compared to EDANS and fourfold higher compared to Mca. The longer-wavelength fluorescence of 5-FAM is less interfered by the autofluorescence and absorbance of drug candidates and cellular components, making the 5-FAM/QXL 520 FRET pair more attractive for use in HTS screening.
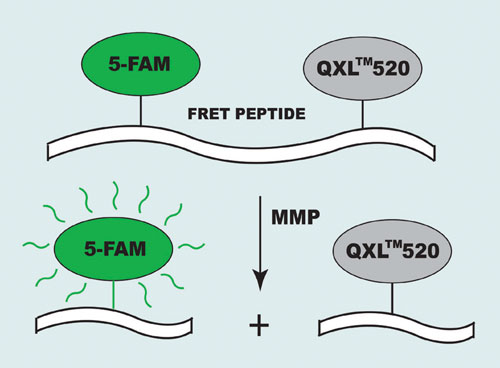
Figure 1
SensoLyte™ 520 MMP Assay Kits
Among the 16 5-FAM/ QXL 520 FRET peptides designed for MMP screening, one peptide showed rapid cleavage by most of the MMPs tested. Using this substrate, AnaSpec developed the SensoLyte 520 Generic MMP Assay Kit for use in measuring the activity of MMP-1, 2, 3, 7, 8, 9, 10, 12, 13, and 14 (Figure 2). This is a homogeneous kit, ideal for high-throughput screening of MMP inducers and inhibitors using purified MMPs. Pro-MMPs enzymes should be activated with 1 mM 4-aminophenylmercuric acetate (APMA) immediately before the experiment and should be activated at a higher protein concentration. After activation, the enzyme can be diluted and used in the assay.
The assay is performed in a convenient 96-well microplate format and takes minimal hands-on time. Activated MMPs are diluted in assay buffer to appropriate concentrations and added to microplate wells with test compounds. The suggested total volume of MMP diluent and test compound for a 96-well plate is 50 µL. The suggested controls for the assay are positive control (MMP enzyme only), inhibitor control (enzyme and known MMP inhibitor), vehicle control (MMP diluent and vehicle used to deliver test compound), test compound control (assay buffer and test compound), and substrate control (assay buffer only).
The generic MMP substrate should be diluted 1:100 in assay buffer and added to the 96-well plate (50 µL/well). For a kinetic reading immediately start measuring fluorescence intensity at Ex/Em=490 nm/520 nm (FlexStation 384II, Molecular Devices(www.moldev.com). Continuously record data every five minutes for 30 to 60 minutes. For an endpoint reading incubate the reaction at room temperature for 30 to 60 min. Keep the plate from direct light. A stop solution can be optionally used (50 µL/well).
The fluorescence reading from the substrate control well represents the background fluorescence. Subtract this background reading from the readings of the other wells to get the fluorescence increase, measured in relative fluorescence units (RFU). For kinetic readings, plot data as RFU versus time for each sample. To convert RFU to concentration of the product of enzymatic reaction, use a fluorescence reference standard provided in the kit. Although cleavage efficiency of generic FRET peptide varies between MMPs, usually the assay can detect the activity of subnanogram quantities of most MMPs.
We compared the sensitivity of 5-FAM/QXL 520 MMP generic substrate and EDANS/DABCYL FRET substrate based on the same sequence using the MMP-13 enzyme. 5-FAM/QXL 520 substrate was cleaved significantly faster than the EDANS/DABCYL substrate (Figure 3). Based on these results, AnaSpec developed a panel of SensoLyte 520 MMP Assay Kits containing long-wavelength 5-FAM/QXL FRET substrates optimized for individual MMPs.
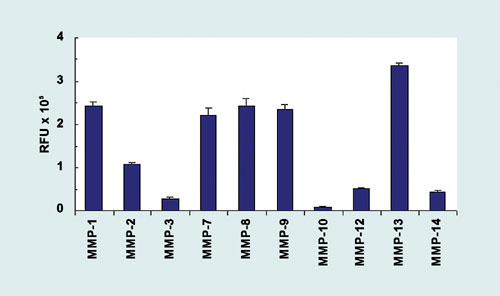
Figure 2
Measurement of MMP Activity in Biological Samples
Most assays for proteases are limited by a lack of specificity. When a FRET-peptide based assay for MMPs is used in cell or tissue lysates, it can be cleaved nonspecifically by several MMPs and other proteases, which are simultaneously present in biological fluids. To detect and quantify the level of activity of a particular MMP in a biological sample, AnaSpec developed a series of SensoLyte Plus 520 MMP assay kits. This assay includes a capture step, using an antibody that immobilizes a particular target MMP from the sample without affecting the enzyme active site and an activity assay step using a 5-FAM/QXL 520 MMP substrate.
mAbs for the specific MMP are coated onto a 96-well plate. These antibodies capture both pro and active forms of MMP enzyme from the mixture. Test samples containing MMPs are added to the microplate wells and incubated for 1 hour at room temperature. Plates are washed and incubated with APMA at a final concentration of 1 mM.
Alternatively, MMPs can be first activated by APMA and then applied to microplate for immunocapture. The plates are washed again and the 5-FAM/QXL 520 FRET substrate solution is added. For end-point reading, the reaction is incubated at room temperature in the dark for 60 min to 16 hours before measurement of fluorescence intensity at Ex/Em=490/520 nm. This assay also can be used to measure MMP activity continuously.
SensoLyte Plus™ 520 MMP assays provide a specific detection of either MMP-1, MMP-9, or MMP-13 activity without crossreactions with another MMP (data not shown). These assays can detect and quantify the presence of subnanogram hMMPs in biological samples.
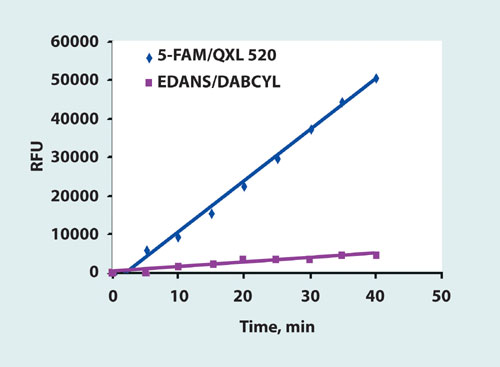
Figure 3
Vera Rakhmanova, Ph.D. ([email protected]), is assay development group manager, Rich Meyer, Ph.D., is manager of R&D, and Xiaohe Tong is peptide development dept. manager at AnaSpec. Web: www.anaspec.com.
Phone: (800) 452-5530