November 1, 2007 (Vol. 27, No. 19)
David Ferrick Ph.D.
Steve Chomicz
Min Wu Ph.D.
Extracellular Flux Assays Quantify Cellular Bioenergetics
Understanding abnormal mitochondrial function in pathophysiology has expanded beyond obvious disorders such as diabetes and obesity into those that include aging, cancer, cardiomyopathy, and neurodegeneration. A common component of these ailments is dysregulation of cellular energy metabolism. Since mitochondria generate the vast majority of cellular ATP via oxidative phosphorylation, they have come under closer scrutiny and, in many indications, are being considered as potential targets for clinical intervention.
In addition to ATP production, mitochondria are responsible for the ß-oxidation of short-, medium-, and long-chain fatty acids as well as central to intermediary metabolism, ROS generation, and apoptosis. Hence, measurement of mitochondrial bioenergetics would provide valuable insight into several disorders and possibly lead to identification of targets for drug discovery.
Traditionally, mitochondrial function has been assessed with Clark-type electrode probes for measuring oxygen consumption, luminescent ATP assays for quantification of total energy metabolism, and MTT or Alamar Blue for determination of metabolic activity.
The Clark electrode provides valuable kinetic information but introduces artifact by its continuous consumption of oxygen, presenting a decreasing oxygen pressure to the cells or isolated mitochondria in the measurement chamber. Although oxygen consumption is a good indicator of mitochondrial function, it only measures one component of cellular bioenergetics and therefore the investigator is oblivious to other pathways that contribute to bioenergetic equilibrium, namely glycolysis.
ATP assays are extremely sensitive but they are not an ideal metric of mitochondrial function as cells strive to maintain a particular ATP budget and will adjust metabolism accordingly. Thus, alterations in ATP levels are usually only detectable during pathophysiological changes.
Artifact has been reported from residual ATP present in dying or dead cells. ATP assays are also destructive and lack kinetic information. Perhaps the two greatest deficiencies of ATP assays are that they do not measure ATP turnover and they cannot determine the relative contribution of energy yielding pathways to total ATP yield.
MTT/XTT and Alamar Blue assays are not as sensitive as ATP assays and have been reported to introduce error through cell toxicity, the very parameter they are supposed to be measuring. Both assays are destructive and lack kinetic information.
Seahorse Bioscience (www.seahorsebio.com) recently introduced a new, label-free, assay system—the XF24 Extracellular Flux Analyzer. Extracellular flux (XF) assays measure the two major energy-producing pathways of the cell simultaneously—mitochondrial respiration (oxygen consumption) and glycolysis (extracellular acidification)—in a sensitive microplate format. XF assays work with adherent cells offering a physiologically relevant, real-time cellular bioenergetic assay.
XF assays also provide comparable performance to biochemical and radioactive methods, with better throughput and without the preparation and use of labels or radioactive materials. Thus, the XF assay format overcomes a lot of the problems and deficiencies of traditional assays that either directly or indirectly measure mitochondrial function. In this tutorial, we will explain how bioenergetic measurements are made using XF and show examples of how it is employed in research and drug discovery.
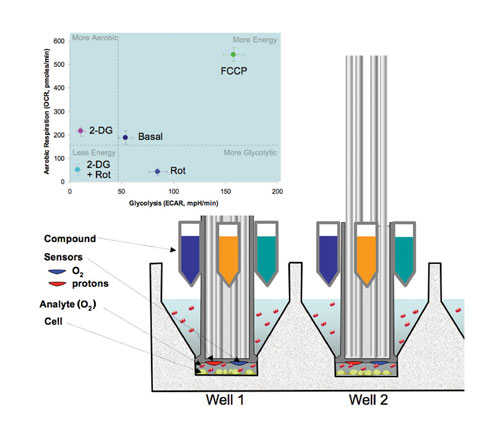
Figure 1
Technology Behind the Physiology
The Seahorse XF24 uses an array of sensors to measure extracellular fluxes of oxygen consumption (OCR) and extracellular acid release (ECAR) from cells in custom microplates (Figure 1). The sensors reside on the ends of 24 plastic probes that comprise the XF sensor cartridge, which fits over the 24-well cell culture plate (Figure 1, cutaway view).
When the sensor cartridge is in the raised position, the O2 and proton sensors are approximately 5 mm above the cells. A measurement under these conditions would take several hours to detect a significant change in oxygen or proton concentrations. In addition, the chamber would have to be sealed to prevent atmospheric oxygen from dissolving into the media as this would disrupt the oxygen gradient formed by the cells.
To speed up the measurement, the sensor cartridge is lowered to approximately 200 µm above the cells, creating a temporary 7 µL microchamber with limited diffusion. The depletion of oxygen or decrease in pH is then measured every 14 seconds for a period of 1.5–4 minutes. Then the cartridge is raised to its original position and the measured media mixes with the much larger portion of the microplate well reservoir to reequilibrate the media. The rate measurements can be made every few minutes without any significant depression of the oxygen tension or acidification of the media.
Four injector ports surrounding the sensor of each well can be used to automatically deliver any combination of pharmacological agents, agonist, antagonists, or substrates during an experiment. Multiple injections of different reagents can be achieved in a single experiment or escalating doses of a single drug can be delivered to generate dose curves in just a few wells. The cartridge is designed to work with 96-well liquid-handling systems. Drug preloading can be achieved by either manual or robotic pipetting.
Plotting OCR and ECAR responses together produces a bioenergetic chart or power grid (Figure 1, top left) indicative of both mitochondrial respiration and glycolysis, respectively.
Substrates and inhibitors are commonly used to probe mitochondrial respiration states. Historically, isolated mitochondria have been employed since many of the substrates used to measure specific components of the respiratory chain do not cross the plasma membrane. Although this approach allows for mechanistic exploration, it is nonphysiologic.
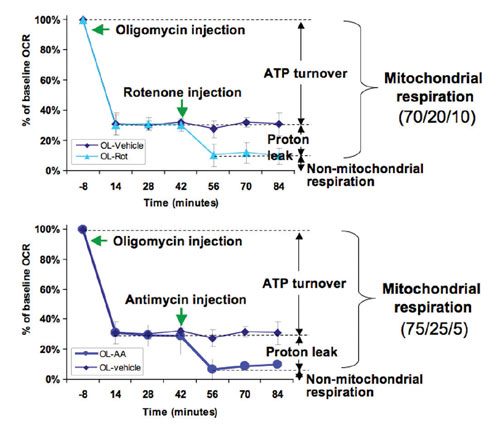
Figure 2
The Mitochondrial Toolbox
Being able to measure mitochondrial function in living cells is necessary as a complement if not a replacement to isolated mitochondria. With XF assays, respiratory function can be assayed using a similar combination of metabolic modulators and substrates that either cross the plasma membrane or are transported into the cell.
In Figure 2, mitochondrial function is measured by mating the microplate containing the intact cells to an XF assay kit sensor cartridge in which the drug delivery chambers have been preloaded with 3 µM oligomycin, 1 µM rotenone, or 1 µM antimycin.
Prior to drug injection, the basal bioenergetic state of the cells is determined by making three or more XF measurements of OCR and ECAR. At this point oligomycin, an inhibitor of complex V ATPase, is automatically injected according to a preprogrammed schedule defined in a Microsoft® Excel™-based assay template that has been loaded on the instrument. The amount of inhibition caused by oligomycin is indicative of the fraction of respiration coupled to ATP production.
Next, either rotenone or antimycin is injected—which blocks complex I or complex III, respectively—and causes a further decrease in OCR. This diminution of OCR is due to that fraction involved in proton leak. At this point, the remaining residual OCR is due to nonmitochondrial respiration.
Mitochondrial respiration capacity could have been determined in the same experiment too had an uncoupler like FCCP been injected (data not shown). Thus, in one experiment changes in mitochondrial function and capacity can be determined in response to drugs, genetic manipulation, and/or pathophysiology.
Since XF assays are label free and nondestructive, the cell plate can be subsequently reused in another assay or placed back into the incubator to make additional measurements at later time points. It is common practice to perform ATP or other viability assays on the same XF cell plate to generate additional information and/or normalize the XF data.
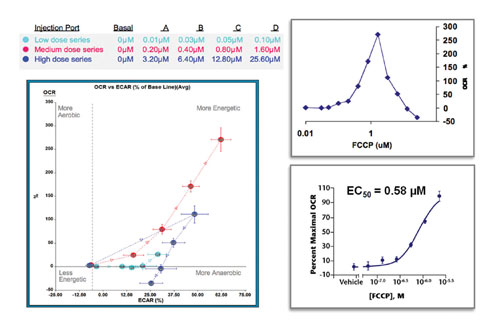
Figure 3
Bioenergetic Profiles
Overnight cultures of HepG2 cells were exposed to increasing concentrations of FCCP, an uncoupler of oxidative phosphorylation, that were injected automatically during the experiment (Figure 3, left panel). By injecting sequentially three dose series of FCCP—low, medium, and high—the minimum and maximal responses were easily determined. As expected both aerobic and glycolytic metabolism was increased to maximal levels to try to overcome the ATP deficit caused by the uncoupler.
In Figure 3 (top right panel), only the mitochondrial component of the data, OCR versus FCCP concentration, was plotted. After the maximal response is reached, higher concentrations become acutely toxic to the cells resulting in decreased OCR and ECAR (Figure 3, left panel), in accordance with other studies on FCCP.
In Figure 3 (bottom right panel), the data is easily converted into a dose response curve that was used to determine the IC50 value. The data for the dose curve is derived from nine wells, three conditions performed in triplicate wells, and the assay was performed in 90 minutes.
XF Assays Have Lower CV’s
It is interesting to note that XF assays often produce data of higher quality than a comparable 96-well assay. For example, in Figure 3 only one concentration of FCCP would be added per well in a traditional 96-well assay format. This would increase the number of wells needed by a factor of four and more as controls for basal activity of the cells are also required. Using the XF format many fewer total wells are used, which reduces the effect of biological variation between cell populations. This translates to lower overall CVs.
In summary, XF assays offer scientists an easy format to measure mitochondrial function in living cells without having to resort to isolating them. The real-time information provides valuable kinetic information not available in endpoint assays, and the multiple parameters measured provide a more global view of bioenergetics.