January 1, 2011 (Vol. 31, No. 1)
CSO Aims to Provide Comprehensive DNA, Protein, and Antibody Manufacturing
The contract service organization (CSO) Aldevron was established in 1998 to offer plasmid DNA production services for the biomanufacturing of vaccines. The company’s two founders, John Ballantyne, Ph.D., now CSO and Michael Chambers, president and CEO, met as students at North Dakota State University (NDSU) in Fargo.
As an undergraduate, Chambers worked on an independent research project about DNA vaccines, which “had recently emerged as a very exciting new technology,” he says. Dr. Ballantyne was a graduate student in pharmaceutical sciences.
DNA vaccines consist of a small, circular piece of bacterial DNA or plasmid, genetically engineered to produce specific antigens of a pathogen. When the company started, DNA vaccine researchers used slow processes and harsh chemicals like cesium chloride to purify plasmid DNA. “We saw an opportunity to manufacture large amounts of DNA on a contract basis,” says Chambers, using a downstream purification process developed at NDSU.
Another problem with early DNA vaccines was that they worked well in mice, but often failed in larger animal and human studies. Aldevron solved the problem by licensing and developing DNA technologies, such as electroporation, and offering them to clients through the company’s GIA™ (Genetic Immunization and Antibody) service. “GIA rescued some DNA vaccines and increased the response to them 100-fold,” says Chambers.
Surprisingly, GIA also proved an effective way to make antibodies. Traditionally, animals are immunized with recombinant proteins or peptides to generate antibodies to a specific antigen. The combination of electroporation and DNA immunization, however, makes it possible to develop antibodies directly to a DNA sequence.
Nucleic acid production remains the flagship service at the Fargo facility, and more than 40,000 projects have been completed for clients working in research, preclinical, clinical, and commercial areas.
Aldevron is headquartered in Fargo and has subsidiaries in Madison, Wisconsin, and Freiburg, Germany. According to Chambers, the acquisition of Genovac in Freiburg in 2004 made it possible to produce high-affinity monoclonal and polyclonal antibodies, including antibodies to complex G-coupled protein receptors, directly from antigen-specific DNA sequences. He explains that the method does not require protein or peptide intermediates. “Our system goes directly from a DNA sequence to a plasmid that can immunize animals and create high-affinity antibodies to difficult antigens. This is Genovac’s expertise,” says Chambers.
The company opened its Madison facility in 2009 to expand its recombinant protein services using mammalian- and insect-cell manufacturing methods. The Aldevron team in Madison has strong expertise in protein purification and assay development as well.
“We now have three contract service platforms at our three sites,” says Tom Foti, vp and GM of Aldevron-Madison. He adds that this gives Aldevron an advantage over competing CSOs that focus only on DNA, protein, or antibody manufacturing. “As an integrated company, we can reduce the vendor base for our clients with the range of services and products we offer,” says Foti, who points out that many clients prefer to deal with one vendor instead of several.
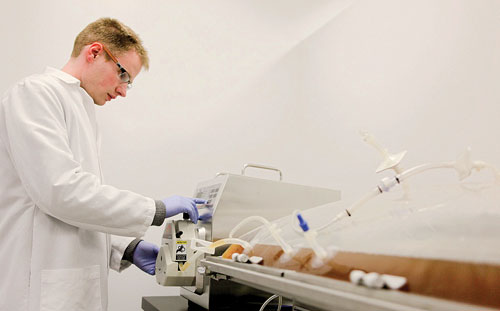
Using novel technology, Aldevron manufactures DNA for a wide range of research, preclinical, clinical, and diagnostic applications.
From West Nile to Spinoffs
When West Nile virus threatened the survival of endangered condors in California, Aldevron was contracted to manufacture a DNA vaccine developed by the Centers for Disease Control and Prevention. “We had production and quality control systems in place that allowed us to manufacture the vaccine in less than two months,” Chambers says. He notes that the vaccine has also been used to protect other endangered species against West Nile virus. This opportunity helped to steer Aldevron into the production of plasmid DNA for clinical trials.
The biomanufacturing services offered by Aldevron fill a variety of needs. Some clients need 10,000 DNA preparations that are one milligram in size, explains Chambers, while others need 100 grams of a single plasmid. He says that Altravax relies on Aldevron to manufacture clinical-grade DNA materials in a streamlined and economic process. Altravax tests multiple vaccine candidates simultaneously to quickly identify the best ones.
Aldevron is currently producing multiple plasmids to support Altravax’ hepatitis B, HIV, and influenza vaccine programs. Altravax also uses Aldevron’s GIA service to screen the thousands of vaccine candidates created through its Molecular Breeding™ technology, Chambers says.
Other companies contract with Aldevron to manufacture control plasmids for use in FDA-approved molecular diagnostic kits or standards for GMP processes and quality control. “We serve the pharmaceutical industry, diagnostic markets, government institutions, and academic researchers,” says Foti.
Chambers says that Aldevron will soon launch its first spinoff company. Researchers at Genovac discovered an antibody that blocks hepatitis C virus infection through a novel mechanism, and workers there are starting a new company built around the technology. “We originally wanted to use profits from contract services to fund the development of new vaccines and therapeutics, and it’s finally playing out.”
Aldevron also started Plasmid.com, which is designed to eliminate routine benchwork associated with processing plasmid maxi-prep kits. The service features a simple website interface and serves as a vitual kit, Chambers asserts. Clients send their plasmid to Aldevron at room temperature, and the sample is scaled up and returned to the client. Chambers says that Plasmid.com is inexpensive, and clients invest no more than 10 minutes of hands-on time. “It’s streamlined and environmentally friendly because it uses less plastic and glassware.”
In addition to supporting its own spinoffs, Aldevron has helped raise $30 million to help its clients expand programs or start related companies. “We take a highly collaborative approach to helping our clients succeed, because we base our success on our client’s success,” says Chambers.